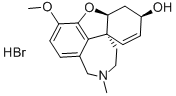
Galanthamine
- CAS NO.:357-70-0
- Empirical Formula: C17H21NO3
- Molecular Weight: 287.35
- MDL number: MFCD00867189
- EINECS: 609-175-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
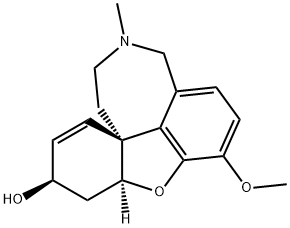
What is Galanthamine?
Absorption
Over a dose range of 8-32 mg/day, galantamine exhibits a dose-linear pharmacokinetic profile. The oral bioavailability of galantamine ranges from 90-100%. Following oral administration, the Tmax is about 1 hour. Following 10 hours of administration, the mean galantamine plasma concentrations were 82–97 μg/L for the 24 mg/day dose and 114–126 μg/L for the 32 mg/day dose.
Toxicity
The oral LD50 of the active ingredient, galantamine hydrobromide, in rats is 75 mg/kg. Symptoms of overdose are expected to be similar to those of cholinomimetics, which involve the central nervous system, the parasympathetic nervous system, and the neuromuscular junction. Effects of a cholinergic crisis include severe nausea, vomiting, gastrointestinal cramping, salivation, lacrimation, urination, defecation, sweating, bradycardia, hypotension, respiratory depression, collapse, and convulsions. Muscle weakness or fasciculations may also occur, with respiratory muscle weakness having the potential to bring fatal results. In one patient who consumed an oral daily dose of 32 mg developed bradycardia, QT prolongation, ventricular tachycardia and torsades de pointes accompanied by a brief loss of consciousness. In one patient with a history of hallucinations who consumed a daily dose of 24 mg galantamine, hallucinations requiring hospitalization occurred. A patient who ingested 160 mg of galantamine from an oral solution developed sweating, vomiting, bradycardia, and near-syncope one hour following consumption.
As in any case of overdose, general supportive measures should be initiated. Tertiary anticholinergics such as intravenous atropine may be used to reverse the cholinergic effects of galantamine. The recommended initial dose of atropine intravenously administered for galantamine overdose ranges from 0.5 to 1.0 mg. It is not known whether galantamine can be removed by dialysis.
Description
Galantamine is an Amaryllidaceae alkaloid which is first obtained from the plant of
snowdrop (Galanthus nivalis), and nowadays, it’s extracted from the plants of
Narcissus and Galanthus species or obtained from chemosynthesis. In many
areas in Europe such as Bulgaria, eastern Turkey, and the Caucasus, the plants of
Galanthus are native species. But its earliest pharmaceutical applications are seldom known. Plaitakis and Duvoisin hypothesized that “moly” in ancient Homer’s
epic might be snowdrops. In Homer’s epic Odyssey, “moly” was used as an antidote
by Odysseus against Circe’s poisonous drugs. To be used as an antidote may be the
plants of Galanthus’ oldest medicinal records. But there is not much evidence for
that. There is little evidence of the traditional application of the plants of
Galanthus, and it is certain that until the Second World War, the plants of Galanthus
and other genera of Amaryllidaceae were not frequently used in European drugs
Italian scholar Iannello studied Pancratium illyricum L., an Amaryllidaceae species endemic to Sardinia, and isolated a new galantamine-type alkaloid in its leaves.
This new galantamine-type compound exhibited a pronounced in?vitro AChE inhibitory activity (IC50? =? 3.5±1.1? μM) in comparison with the reference standard
galantamine hydrobromide (IC50?=?1.5±0.2?μM)
Chemical properties
Off-White Solid
Physical properties
Hydrobromide of galantamine can be used as drug although galantamine can’t be
used as drugs
Appearance: an almost white powder. Solubility: soluble in water; slightly soluble in alcohol; and insoluble in acetone, trichloromethane, and diethyl ether.
Melting point: 269–270?°C. Specific optical rotation: ?90 to ?100°.
Originator
Reminyl,Janssen Pharmaceutica Inc.
Occurrence
Galanthamine is a bulb plant found throughout the world.
History
In the early 1950s, Soviet Union’s scientists started modern medicine research of
galantamine. In 1951, the Soviet Union’s pharmacologist Mashkovsky and
Kruglikova-Lvova firstly proved its AChE-inhibiting properties, which was published in the paper. In 1957, the Bulgarian pharmacologist Paskov et?al. found that
the plants of Galanthus were the richest sources of galantamine, which opened a
way for its commercial development by the company Sopharma in Bulgaria.
Galantamine hydrobromide was launched into market under the trade name Nivalin.
Initially, Nivalin was used in anesthesia to antagonize the effects of non-depolarizing
muscle relaxants, and since then, it was rapidly introduced in other areas of
medicine
Galantamine hydrobromide was launched into market for the indication of mild
to moderate Alzheimer’s disease firstly in 1995, developed by Sanochemia
Pharmaceuticals. In the United States, galantamine hydrobromide was developed
by Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, and was listed
under the trade name Razadyne in 2001. So far, galantamine hydrobromide has
been marketed in more than 20 countries and regions, such as the United States,
Europe, and Japan.
The Uses of Galanthamine
A selective acetylcholinesterase inhibitor. Useful for the treatment of Alzheimer's disease.
The Uses of Galanthamine
synthetic fluoroquinolone antibiotic agent
The Uses of Galanthamine
Galantamine is approved for the treatment of mild-to-moderate AD.It is a reversible specific inhibitor of AChE,and in addition it modulates central nicotinic receptors toincrease cholinergic neurotransmission.
Background
Galantamine is a tertiary alkaloid and reversible, competitive inhibitor of the acetylcholinesterase (AChE) enzyme, which is a widely studied therapeutic target used in the treatment of Alzheimer's disease. First characterized in the early 1950s, galantamine is a tertiary alkaloid that was extracted from botanical sources, such as Galanthus nivalis. Galantamine was first studied in paralytic and neuropathic conditions, such as myopathies and postpolio paralytic conditions, and for reversal of neuromuscular blockade. Following the discovery of its AChE-inhibiting properties, the cognitive effects of galantamine were studied in a wide variety of psychiatric disorders such as mild cognitive impairment, cognitive impairment in schizophrenia and bipolar disorder, and autism; however, re-development of the drug for Alzheimer’s disease did not commence until the early 1990s due to difficulties in extraction and synthesis. Galantamine blocks the breakdown of acetylcholine in the synaptic cleft, thereby increasing acetylcholine neurotransmission. It also acts as an allosteric modulator of the nicotinic receptor, giving its dual mechanism of action clinical significance.
The drug was approved by the FDA in 2001 for the treatment of mild to moderate dementia of the Alzheimer's type. As Alzheimer's disease is a progressive neurodegenerative disorder, galantamine is not known to alter the course of the underlying dementing process. Galantamine works to block the enzyme responsible for the breakdown of acetylcholine in the synaptic cleft, thereby enhancing cholinergic neuron function and signalling. Under this hypothesized mechanism of action, the therapeutic effects of galantamine may decrease as the disease progression advances and fewer cholinergic neurons remain functionally intact. It is therefore not considered to be a disease-modifying drug. Galantamine is marketed under the brand name Razadyne, and is available as oral immediate- and extended-release tablets and solution.
What are the applications of Application
Galanthamine is a selective acetylcholinesterase inhibitor
What are the applications of Application
Galanthamine hydrochloride is a selective inhibitor of AChE (acetylcholinesterase)
Indications
Galantamine is indicated for the treatment of mild to moderate dementia of the Alzheimer’s type.
Definition
ChEBI: A natural product found in Crinum asiaticum var. sinicum.
Indications
Its formulation includes capsule, tablet, and oral solution. It is used in the symptomatic treatment of mild to moderately severe dementia in Alzheimer’s disease.
Manufacturing Process
2 Methods of isolation of galantamine from Narcissus pseudonarcissus bulbs
1. 10 kg air-dried, comminuted bulbs of Narcissus pseudonarcissus "Carlton" is carefully mixed with 400 g sodium carbonate. 23 L dichloroethane is added.
The mixture is allowed to stand for 10 h; then the solvent is decanted. The
bulbs are once again doused with 23 L dichloroethane which is decanted after
2 to 3 hs. After that, 17 L dichloroethane is added to the bulbs for the third
time; however, this is decanted immediately. The mixed dichloroethane
extracts are extracted by means of 10% sulfuric acid (2 times 600 ml each; 2
times 300 ml each).
The acidic extracts are mixed and purified from traces of dichloroethane by
means of shaking out with diethyl ether. Under stirring and cooling to 15° to
20°C, about 200 ml of a 25% aqueous ammonia solution is then added up to
alkaline litmus reaction (the pH is in the range of 7 to 8). Different from the
indications in the art, the companion alkaloids do not precipitate. The alkaline
solution is saturated with salt and extracted with diethyl ether.
After evaporation of the ether, a negligible residue remains, which is also
different from the indications in the art. The pH-value of the aqueous phase is
set to about 14 by saturating it with potash. The aqueous phase is repeatedly
extracted with diethyl ether. The mixed ether extracts are evaporated to
dryness, the remaining galanthamine-containing residue is dissolved in
acetone (50 ml). In contrast to the art, there is no precipitate. 350 ml
acetone is replenished, 200 g aluminum oxide is added, and stirring is
effected for 45 min. The aluminum oxide is filtered off and washed twice with
100 ml acetone each time. The mixed acetone solutions are evaporated to
dryness. 1.3 g of an oily residue is obtained which is examined by means of
HPLC.
2. 100 kg air-dried, comminuted bulbs of Narcissus pseudonarcissus "Carlton"
is carefully mixed with 4 kg of sodium carbonate. The mixture is divided into
three equal parts, and each is doused with 15 L special boiling-point gasoline
80/110. The mixtures are allowed to stand for 24 hs. The solvents are each
renewed twice, collected, and evaporated to dryness in low vacuum. The
extracts are placed in 2% aqueous sulfuric acid and adjusted to a pH of 4 with
concentrated aqueous ammonia solution. Five extractions with diethyl ether
follow. The aqueous phase is set to a pH of 9 with concentrated ammonia and
extracted five times with diethyl ether. These ether fractions are collected,
dried with sodium sulfate, and evaporated. 20 g of a slightly yellow, oily
residue is obtained, which is recrystallized from hot isopropanol. 10 g of white
galanthamine base having a melting point of 129°-130°C is obtained.
Galantamine may be isolated from Galanthus nivalis or G. woronowi bulbs too.
Therapeutic Function
Cholinesterase inhibitor
General Description
Galantamine, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6H-benzofuro-[3a,3,2,ef][2]-benzazepin-6-ol (Nivalin, Reminyl), is an alkaloid extractedfrom the tuberous plant Leucojum aestivum (L.) belongingto the Amaryllidaceae family and from the bulbs of thedaffodil, Narcissus pseudonarcissus. It is a reversiblecholinesterase inhibitor that appears to have no effect on butyrylcholinesterase.In addition, it acts at allosteric nicotinicsites, further enhancing its cholinergic activity. Galantamineundergoes slow and minor biotransformation with approximately5% to 6% undergoing demethylation. It is primarilyexcreted in the urine.
Pharmacokinetics
Galantamine is a competitive and reversible inhibitor of acetylcholinesterase that works to increase acetylcholine levels. Galantamine acts both centrally and peripherally to inhibit both muscle and brain acetylcholinesterase, thereby increasing cholinergic tone. Galantamine is also a positive allosteric modulator of neuronal nicotinic acetylcholine receptors. As dementia is a progressive neurodegenerative disease, galatamine has a negligible effect in altering the course of the underlying process of dementia and may exert its therapeutic effectiveness for a short period of time. However, galantamine promoted improvements in cognition, global function, activities of daily living, and behavioural symptoms in clinical studies of Alzheimer’s disease.
Galantamine exhibited therapeutic efficacy in studies of vascular dementia and Alzheimer’s disease with cerebrovascular disease. In one study, galantamine reversed scopolamine-induced acute anticholinergic syndrome that was characterized by drowsiness, disorientation, and delirium.
Pharmacology
hydrolyzed and metabolized by AChE.?When AD occurs, neurons in the basal forebrain region of the brain are lost, resulting in the reduction of synthesis, storage and
release of ACh, which leads to a variety of clinical manifestations especially memory and recognition dysfunction. Galantamine is a reversible AChE inhibitor, which
is highly selective and competitive. It competitively binds to AChE in a synaptic gap
with Ach, blocks the degradation of Ach by AChE, and thus achieves efficacy.
Besides, it has the activity as an allosteric modulator of nicotinic acetylcholine
receptor (nAChR). nAChR is mainly located in the muscarinic choline receptor’s
presynaptic structure. It can promote the release of Ach, which is critical to maintain
the survival and function of cholinergic neurons. The study found that in cerebral cortex of the patients of AD, nAChR was significantly reduced. Galantamine binds
to AChR’s allosteric site to increase the transmission of acetylcholine signaling.
Galantamine has a 53-fold greater inhibitory activity in?vitro for AChE than for
butyrylcholinesterase (BChE). The low affinity of galantamine for BChE may be
responsible for a tolerability advantage. BChE, which is primarily found in plasma,
is not thought to be directly involved in the cholinergic disruption seen in early AD,
and its inhibition may contribute to peripheral side effects.
It was recorded in Martindale: The Complete Drug Reference that an initial oral
dose of 4?mg is given twice daily with food for 4?weeks and then increased to 8?mg
twice daily. This dose should be maintained for at least 4?weeks; thereafter, the dose
may be further increased to 12?mg twice daily according to response and tolerance.
The clinical benefit of galantamine should be reassessed, preferably within the first
3?months and thereafter on a regular basis
Galantamine is metabolized by hepatic cytochrome P450 enzymes CYP3A4 and
CYP2D6, glucuronidated, and excreted unchanged in the urine. After intravenous
injection or oral administration, about 20% of the dose was excreted as unchanged
galantamine in the urine, and the other was metabolized by the hepar.
Clinical Use
Before the 1990s, galantamine was mainly used to treat poliomyelitis sequelae,
muscle atrophy, postoperative intestinal muscle paralysis, urinary retention, and
myasthenia gravis. In the 1990s, it was found that galantamine had improved memory impairment in mice, suggesting that it might be effective for central cholinergic
disorders in Alzheimer’s disease
In a 3–6?months’ well-designed clinical trial, recipients of galantamine achieved
significant improvements in cognitive symptoms compared to placebo recipients.
Galantamine also improved activities of daily living in these patients and significantly reduced the requirement for caregiver assistance with activities of daily living. Clinical development of new indications of galanthamine is currently
underway, such as smoking cessation and improving cognitive impairment in
schizophrenia and mania.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: erythromycin increases concentration
of galantamine.
Metabolism
In vitro study findings suggest that about 75% of the drug is metabolized by CYP2D6 and CYP3A4. CYP2D6 promotes O-demethylation of the drug to form O-desmethyl-galantamine and the CYP3A4-mediated pathway forms the galantamine-N-oxide. Important metabolic pathways also include N-demethylation, epimerization, and sulfate conjugation. Other metabolites include norgalantamine, O-desmethyl-galantamine, O-desmethyl-norgalantamine, epigalantamine and galantaminone, which do not retain clinically significant pharmacology activities.
Galantamine can also undergo glucuronidation: in one oral radiolabeled drug study in poor and extensive CYP2D6 metabolizers, about 14-24% of the total radioactivity was identified as galantamine glucuronide 8 hours post-dose. O-demethylation by CYP2D6 becomes prominent in patients with who are extensive metabolizers of CYP2D6, but unchanged galatamine (39-77%) and its glucuronide metabolite (14-24%) predominated in the plasma of both poor and extensive metabolizers of CYP2D6 in a radiolabelled drug study. The total plasma clearance, or nonrenal clearnace, accounts for 20–25% of drug elimination.
Metabolism
Galantamine is partially (up to 75%) metabolised by the
cytochrome P450 isoenzymes CYP2D6 and CYP3A4; a
number of active metabolites are formed.
After 7 days, 90-97% of a single oral dose is recovered
in the urine with up to about 6% detected in the faeces;
about 20-30% of the dose is excreted in the urine as
unchanged galantamine. Clearance is reported to be 20%
lower in females than in males and 25% lower in poor
metabolisers than in extensive metabolisers.
Properties of Galanthamine
| Melting point: | 119-1210C |
| Boiling point: | 429.65°C (rough estimate) |
| alpha | D20 -118.8° (c = 1.378 in ethanol) |
| Density | 1.0662 (rough estimate) |
| refractive index | 1.5022 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | ≥14.37 mg/mL in DMSO; ≥14.43 mg/mL in H2O with gentle warming; ≥45 mg/mL in EtOH with gentle warming |
| form | Powder |
| pka | pKa 8.32 (Uncertain) |
| color | White to off-white |
| CAS DataBase Reference | 357-70-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Galantamin(357-70-0) |
Safety information for Galanthamine
Computed Descriptors for Galanthamine
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran


You may like
-
 357-70-0 Galantamine 99%View Details
357-70-0 Galantamine 99%View Details
357-70-0 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Phenylephrine HCl 61-76-7 98%View Details
Phenylephrine HCl 61-76-7 98%View Details
61-76-7 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3 -
 Abiretorone 154229-18-2 98%View Details
Abiretorone 154229-18-2 98%View Details
154229-18-2 -
 73590-58-6 Omeprazole 98%View Details
73590-58-6 Omeprazole 98%View Details
73590-58-6 -
 201530-41-8 Deferasirox 98%View Details
201530-41-8 Deferasirox 98%View Details
201530-41-8 -
 Sertraline HCl 98%View Details
Sertraline HCl 98%View Details
79559-97-0
