CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Crystals from benzene |
| Melting Point | 126-127 °C |
| Solubility | Crystals from water; decomposition 256-257 °C. Sparingly sol in cold; more sol in hot water. Very sparingly sol in alcohol, acetone. /Hydrochloride/ |
| LogP | 1.8 |
| Optical Rotation | Crystals from water; dec 246-247 °C. Specific optical rotation = -93.1 deg at 20 °C/D ( c = 0.1015 g/100 ml in 15 mL H2O) /Hydrobromide/ |
| Dissociation Constants | 8.32 |
| Collision Cross Section | 166.9 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)] |
| Other Experimental Properties | Monoacidic base |
COMPUTED DESCRIPTORS
| Molecular Weight | 287.35 g/mol |
|---|---|
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 287.15214353 g/mol |
| Monoisotopic Mass | 287.15214353 g/mol |
| Topological Polar Surface Area | 41.9 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 440 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
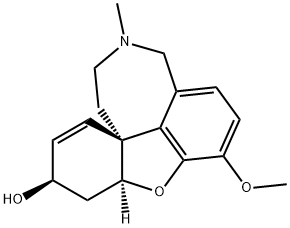
Galanthamine is a benzazepine alkaloid isolated from certain species of daffodils. It has a role as an antidote to curare poisoning, an EC 3.1.1.7 (acetylcholinesterase) inhibitor, a cholinergic drug, an EC 3.1.1.8 (cholinesterase) inhibitor and a plant metabolite. It is an organic heterotetracyclic compound, a tertiary amino compound, a benzazepine alkaloid and a benzazepine alkaloid fundamental parent. It is a conjugate base of a galanthamine(1+).