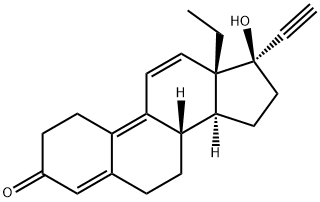
Formestane
Synonym(s):4-Hydroxyandrost-4-ene-3,17-dione;Aromatase;CYPXIX;Estrogen synthetase;
- CAS NO.:566-48-3
- Empirical Formula: C19H26O3
- Molecular Weight: 302.41
- MDL number: MFCD00057814
- EINECS: 625-331-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-29 17:45:46
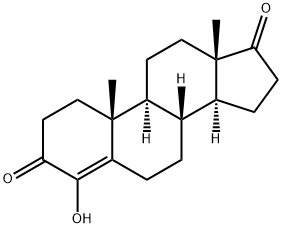
What is Formestane?
Absorption
Formestane has poor oral bioavailability, but is fully bioavailable when administered via the established intramuscular route. The AUC after an intravenous pulse dose does not vary considerably from that of an intramuscular dose.
Within 24-48 h of the first dose of intramuscular formestane, a C(max) of 48.0 +/- 20.9 nmol/l was achieved in one study. [2]
Description
Formestane is a potent aromatase inhibitor launched in the UK as a second-line endocrine treatment for breast cancer. As a synthetic derivative of androstanedione, the natural substrate for the biosynthesis of estrogen by the enzyme aromatase, formastane selectively inhibits aromatase and binds to its steroid receptor site to cause a rapid and sustained fall in circulating estrogen level and, therefore, inhibits tumor growth. In patients with existing bulky primary tumors, formestane effectively reduces the size of the tumors. Formastane has apparent tolerability advantages and less side effects than other agents such as aminoglutethimide.
Description
4-hydroxy Androstenedione (4-HAD) is a steroidal inhibitor of aromatase (also known as cytochrome P450 19A1; Ki = 27 nM). As aromatase catalyzes the conversion of androgens to estrogens, aromatase inhibitors, including 4-HAD, are used against hormone-sensitive breast cancer in menopausal women. They are also abused in combination with anabolic steroids in racehorses and athletes. This product is intended for forensic and research applications.
Chemical properties
Needles
Originator
Ciba-Geigy (Switzerland)
History
Formestane is a second-generation steroidal aromatase inhibitor, and the first one to reach clinical use during the early 1990s[1].
The Uses of Formestane
An antitumor drug. An aromatase inhibitor
The Uses of Formestane
antineoplastic, aromatase inhibitor
The Uses of Formestane
An antitumor drug. An aromatase inhibitor.
Indications
For the treatment of estrogen-receptor positive breast cancer in post-menopausal women.
Background
Formestane was the first selective, type I, steroidal aromatase inhibitor used in the treatment of estrogen-receptor positive breast cancer in post-menopausal women. Formestane suppresses estrogen production from anabolic steroids or prohormones. Formestane is also a prohormone of 4-hydroxytestosterone, an active steroid with weak androgenic activity and mild aromatase inhibitor activity. It is listed as a prohibited substance by the World Anti-Doping Agency for use in athletes.
Formestane has poor oral bioavailability, and thus must be administered fortnightly (bi-weekly) by intramuscular injection. Some clinical data has suggested that the clinically recommended dose of 250mg was too low. With the discovery of newer, non-steroidal and steroidal, aromatase inhibitors which were orally active and less expensive than formestane, formestane lost popularity.
Currently, formestane (categorized as an anti-estrogenic agent) is prohibited from use in sports in accordance to the regulations of the World Anti-Doping Agency. It is not US FDA approved, and the intramuscular injection form of formestane (Lentaron) which was approved in Europe has been withdrawn.
Definition
ChEBI: A 17-oxo steroid that is androst-4-ene-3,17-dione in which the hydrogen at position 4 is replaced by a hydroxy group. Formestane was the first selective, type I steroidal aromatase inhibitor, suppressing oestrogen production from anabolic steroids or proho mones. It was formerly used in the treatment of oestrogen-receptor positive breast cancer in post-meopausal women. As it has poor oral bioavailability, it had to be administered by (fortnightly) intramuscular injection. It fell out of use with the subseque t development of cheaper, orally active aromatase inhibitors. Formestane is listed by the World Anti-Doping Agency as a substance prohibited from use by athletes.
brand name
Lentaron
Mechanism of action
Aromatase inhibitors such as formestane and letrozole reduce plasma estradiol levels by inhibiting the conversion of testosterone to estrogen. This compound was first described as a competitive inhibitor, but subsequent evidence proved that its binding to aromatase was irreversible. Hence, it is a suicide inhibitor. In the dog, but not rodents, this results in Leydig cell hypertrophy and hyperplasia. This is thought to be due to the differential sensitivity of the pituitary feedback mechanism to estrogens and androgens in the different species[1-2].
Pharmacokinetics
By significantly reducing estrogen levels in the bloodstream, formestane may exhibit antitumor activity.
In one trial involving 147 postmenopausal females with advanced breast cancers resistant to standard therapies, 22% of patients achieved a partial response, while another 20% achieved disease stabilization. [3]
In comparative trials comparing a non-steroidal aromatase inhibitor, anastrozole, with formestane, it was found that anastrozole was more effective and consistent at suppressing estrogen levels in the body. However, these results were of unverified clinical significance. [5]
Metabolism
Hepatic metabolism. Phase I of metabolism is mainly reductive in nature. The reduction products 3 beta-hydroxy-5alpha-androstane-4,17-dione and 3alpha-hydroxy-5beta-androstane-4,17-dione are produced, and further reduced.
A notable step in the process of metabolism is a keto reduction on carbon number three of the molecule. The main metabolite which is produced from formestane is 4-hydroyxyandrost-4-ene-3,17-dione-4-glucuronide.
The oxidation products identified were 4-hydroxyandrosta-4,6-diene-3,17-dione and 4-hydroxyandrosta-1,4-diene-3,17-dione.
In phase II, conjugation was diverse and included sulfatation and glucuronidation. 4-hydroxytestosterone, the 17-hydroxylated analog to formestane, was identified as one particular metabolite found in women's urine. This finding was the result of an oral administration of 500mg of formestane in women.
References
[1] Avenda?o C, et al. Anticancer Drugs That Inhibit Hormone Action. Medicinal Chemistry of Anticancer Drugs, 2008; 53-91.0
[2] Berczi I, et al. Biologically Controlled Mutations are Right for Evolution. Insights to Neuroimmune Biology, 2016; 217-241.
Properties of Formestane
| Melting point: | 199-202°C |
| Boiling point: | 383.44°C (rough estimate) |
| alpha | D20 +181° (c = 7.7 in chloroform) |
| Density | 1.1189 (rough estimate) |
| refractive index | 1.4200 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | solid |
| pka | 9.31±0.60(Predicted) |
| color | White to Off-White |
| InChI | InChI=1S/C19H26O3/c1-18-10-8-15(20)17(22)14(18)4-3-11-12-5-6-16(21)19(12,2)9-7-13(11)18/h11-13,22H,3-10H2,1-2H3/t11-,12-,13-,18+,19-/m0/s1 |
| CAS DataBase Reference | 566-48-3(CAS DataBase Reference) |
Safety information for Formestane
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Formestane
| InChIKey | OSVMTWJCGUFAOD-KZQROQTASA-N |
| SMILES | C1(=O)C(O)=C2[C@](C)(CC1)[C@]1([H])[C@]([H])([C@@]3([H])[C@@](CC1)(C)C(=O)CC3)CC2 |
Formestane manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Formestane CAS 566-48-3View Details
Formestane CAS 566-48-3View Details
566-48-3 -
 Formestane (Lentaron) Powder, 50kg BagView Details
Formestane (Lentaron) Powder, 50kg BagView Details
566-48-3 -
 Formestane Lentaron PowderView Details
Formestane Lentaron PowderView Details
566-48-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
