566-48-3
CHEMICAL AND PHYSICAL PROPERTIES
| Melting Point | 199 - 202 |
|---|---|
| Solubility | Insoluble |
| LogP | 2.66 |
| Dissociation Constants | 9.31 |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 302.4 g/mol |
|---|---|
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 302.18819469 g/mol |
| Monoisotopic Mass | 302.18819469 g/mol |
| Topological Polar Surface Area | 54.4 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 590 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
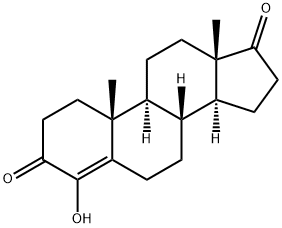
Formestane is a 17-oxo steroid that is androst-4-ene-3,17-dione in which the hydrogen at position 4 is replaced by a hydroxy group. Formestane was the first selective, type I steroidal aromatase inhibitor, suppressing oestrogen production from anabolic steroids or prohormones. It was formerly used in the treatment of oestrogen-receptor positive breast cancer in post-meopausal women. As it has poor oral bioavailability, it had to be administered by (fortnightly) intramuscular injection. It fell out of use with the subsequent development of cheaper, orally active aromatase inhibitors. Formestane is listed by the World Anti-Doping Agency as a substance prohibited from use by athletes. It has a role as an EC 1.14.14.14 (aromatase) inhibitor and an antineoplastic agent. It is a 3-oxo-Delta(4) steroid, a 17-oxo steroid, a hydroxy steroid and an enol. It derives from a hydride of an androstane.