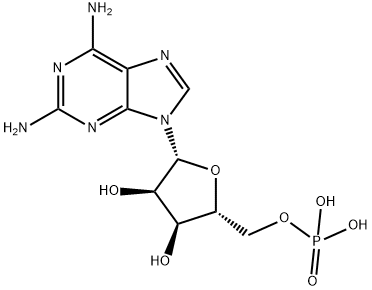
Fludarabine phosphate
Synonym(s):2-Fluoro-9-(5-O-phosphono-β-D -arabinofuranosyl)-9H-purin-6-amine;Fludarabine phosphate
- CAS NO.:75607-67-9
- Empirical Formula: C10H13FN5O7P
- Molecular Weight: 365.21
- MDL number: MFCD00866418
- EINECS: 616-242-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
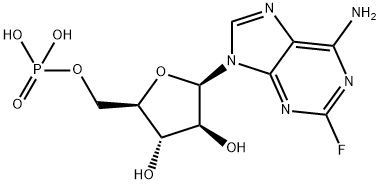
What is Fludarabine phosphate?
Description
Fludarabine phosphate is an antimetabolite indicated for the treatment of B cell lymphocytic leukemia. It is reportedly effective in patients refractory to other therapies. Fludarabine phosphate acts by inhibiting primer RNA synthesis. Its side effects include bone marrow suppression, anemia, thrombocytopenia and neutropenia.
Chemical properties
White or almost white, crystalline powder, hygroscopic.
Originator
Southern Research Institute (U.S.A.)
The Uses of Fludarabine phosphate
Fludarabine phosphate is used for the treatment of chronic lymphatic leukemia and low-grade lymphoma. In the circulation,fludarabine phosphate is immediately dephosphorylated to the nucleoside fludarabine. About 30-40% of nucleoside fludarabine is excreted into the urine. In addition, fludarabine is metabolized into a hypoxanthine metabolite also excreted in the urine.Intracellularly,fludarabine is stepwise rephosphorylated to the active triphosphate. Deoxycytidine kinase is the dominant, if not the exclusive,enzyme for the formation of the monophosphate. Adenylate kinase and nucleoside diphosphate kinase are believed to be involved in the formation of the diphosphate and triphosphate,respectively.
The Uses of Fludarabine phosphate
anticonvulsant
Definition
ChEBI: Fludarabine phosphate is a purine arabinonucleoside monophosphate having 2-fluoroadenine as the nucleobase. A prodrug, it is rapidly dephosphorylated to 2-fluoro-ara-A and then phosphorylated intracellularly by deoxycytidine kinase to the active triphosphate, 2-fluoro-ara-ATP. Once incorporated into DNA, 2-fluoro-ara-ATP functions as a DNA chain terminator. It is used for the treatment of adult patients with B-cell chronic lymphocytic leukemia (CLL) who have not responded to, or whose disease has progressed during, treatment with at least one standard alkylating-agent containing regimenas. It has a role as an antimetabolite, an antineoplastic agent, an immunosuppressive agent, an antiviral agent, a prodrug and a DNA synthesis inhibitor. It is an organofluorine compound, a nucleoside analogue and a purine arabinonucleoside monophosphate. It derives from a 2-fluoroadenine.
brand name
Fludara (Berlex), Benefluor (Schering AG).
Mechanism of action
Fludarabine phosphate is a cytotoxic purine antimetabolite that acts by inhibiting DNA synthesis. Fludarabine and its soluble derivatives interfere with phosphorylation, e.g., in L 1210 cells. Fludarabine behaves more like an analog of deoxycytidine than adenine or deoxyadenine as indicated by reports demonstrating that the presence of fluorine in the 2-position of the adenine ring alters its function as a substrate for deaminase and nucleoside kinases. This results in differences in biological activity and metabolism. Halogenation does not simply block deamination, but also influences the enzyme that carries out the phosphorylation, as a result cytotoxicity is increased. Fludarabine phosphate may selectively inhibit the incorporation of thymidine and uridine into the DNA molecule by inhibiting both ribonucleotide reductase and DNA polymerase. The maximum tolerated dose (MTD) in heavily pretreated patients with advanced malignancy/solid tumors on the daily regimen was about 15 mg/m2. Granulocytopenia and thrombocytopenia were dose-limiting.
Pharmacology
Fludarabine phosphate is rapidly dephosphorylated to 2-fluoro-ara-A and then phosphorylated intracellularly by deoxycytidine kinase to the active triphosphate, 2-fluoro-ara-ATP. This metabolite appears to act by inhibiting DNA polymerase alpha, ribonucleotide reductase and DNA primase, thus inhibiting DNA synthesis. The mechanism of action of this antimetabolite is not completely characterized and may be multi-faceted.
Phase I studies in humans have demonstrated that fludarabine phosphate is rapidly converted to the active metabolite, 2-fluoro-ara-A, within minutes after intravenous infusion.
Consequently, clinical pharmacology studies have focused on 2-fluoro-ara-A pharmacokinetics. After the five daily doses of 25 mg 2-fluoro-ara-AMP/m2 to cancer patients infused over 30 minutes, 2-fluoro-ara-A concentrations show a moderate accumulation. During a 5-day treatment schedule, 2-fluoro-ara-A plasma trough levels increased by a factor of about 2. The terminal half-life of 2-fluoro-ara-A was estimated as approximately 20 hours. In vitro, plasma protein binding of fludarabine ranged between 19% and 29%.
Clinical Use
Fludarabine phosphate (Fludara ® ), is a fluorinated nucleotide analog of the antiviral agent vidarabine, 9-β-D-arabinofuranosyladenine(ara-A), which differs only by the presence of a fluorine atom at position 2 of the purine moiety and a phosphate group at position 5 of the arabinose moiety (Plunkett et al., 1993). These structural modifications result in increased aqueous solubility and resistance to enzymatic degradation by adenosine deaminases compared to vidarabine (Brockman et al., 1977; Plunkett et al., 1990). Fludarabine phosphate is indicated for the treatment of patients with B-cell chronic lymphocytic leukemia (CLL) who have not responded to or whose disease has progressed during treatment with at least one standard alkylating agent containing regimen (Boogaerts et al., 2001; Rossi et al., 2004).
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid concomitant use with
clozapine, increased risk of agranulocytosis.
Cytotoxics: increased pulmonary toxicity with
pentostatin (unacceptably high incidence of
fatalities); increases intracellular concentration of
cytarabine.
Metabolism
Intravenous fludarabine phosphate is rapidly dephosphorylated to fludarabine which is taken up by lymphocytes and rephosphorylated via the enzyme deoxycytidine kinase to the active triphosphate nucleotide. Clearance of fludarabine from the plasma is triphasic; elimination is mostly via renal excretion: 40-60% of an intravenous dose is excreted in the urine. The pharmacokinetics of fludarabine show considerable inter-individual variation
Properties of Fludarabine phosphate
| Melting point: | 203°C(dec.)(lit.) |
| Boiling point: | 864.2±75.0 °C(Predicted) |
| alpha | [α]D20 +10~+14゜(c=0.5,H2O) |
| Density | 2.39±0.1 g/cm3(Predicted) |
| RTECS | UO7440900 |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble1mg/mL |
| form | Powder |
| pka | 1.86±0.10(Predicted) |
| color | white |
| Water Solubility | Soluble in DMSO or water at 5mg/ml |
| Merck | 14,4126 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 75607-67-9(CAS DataBase Reference) |
Safety information for Fludarabine phosphate
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Fludarabine phosphate
| InChIKey | GIUYCYHIANZCFB-GFRUICAKSA-N |
Fludarabine phosphate manufacturer
New Products
Tetrabutylammonium hydrogen sulfate 2,2,6-Trimethyl-4H-1,3-dioxin-4-one 4-Piperidinopiperidine tert-Butyl acetoacetate 4-BROMOMETHYLTETRAHYDROPYRAN 3-Hydroxyazetidine hydrochloride Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) 3-Methoxybenzonitrile 4-Methoxybenzonitrile Dibenzoyl Peroxide Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran

You may like
-
 75607-67-9 Fludarabine phosphate 98%View Details
75607-67-9 Fludarabine phosphate 98%View Details
75607-67-9 -
 75607-67-9 98%View Details
75607-67-9 98%View Details
75607-67-9 -
 75607-67-9 Fludarabine phosphate 98%View Details
75607-67-9 Fludarabine phosphate 98%View Details
75607-67-9 -
 Fludarabine Monophosphate CAS 75607-67-9View Details
Fludarabine Monophosphate CAS 75607-67-9View Details
75607-67-9 -
 Fludarabine phosphate 97% CAS 75607-67-9View Details
Fludarabine phosphate 97% CAS 75607-67-9View Details
75607-67-9 -
 Fludarabine phosphate CAS 75607-67-9View Details
Fludarabine phosphate CAS 75607-67-9View Details
75607-67-9 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4
