Milrinone
Synonym(s):1,6-Dihydro-2-methyl-6-oxo-(3,4ʹ-bipyridine)-5-carbonitrile;1,6-Dihydro-2-methyl-6-oxo-(3,4′-bipyridine)-5-carbonitrile;Milrinone - CAS 78415-72-2 - Calbiochem
- CAS NO.:78415-72-2
- Empirical Formula: C12H9N3O
- Molecular Weight: 211.22
- MDL number: MFCD00133539
- EINECS: 278-903-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 18:23:36
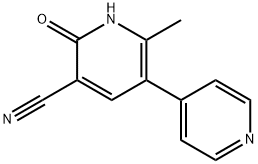
What is Milrinone?
Absorption
When administered as an IV bolus dose of 10-100 μg/kg, milrinone induces hemodynamic effects within 60 seconds reaching a peak effect by 2-5 minutes. The plasma AUC is significantly dose-dependent.
Toxicity
Toxicity information regarding milrinone is not readily available. Patients experiencing an overdose are at an increased risk of severe adverse effects such as hypotension and adverse cardiac events such as ventricular arrhythmias. Symptomatic and supportive measures are recommended.
Description
Milrinone, an inhibitor of phosphodiesterase selective for fraction III PDE, exerts a positive inotropic effect on the heart as well as a peripheral vasodilatory effect. Milrinone given intravenously produces significant improvements on cardiac output, pulmonary capillary wedge pressure and vascular resistance without significant effects on the heart rate.
Description
Milrinone is an inhibitor of type 3 phosphodiesterases (PDEs), inhibiting recombinant PDE3A and PDE3B with IC50 values of 0.45 and 1 μM, respectively. It is selective for PDE3 over PDE1, PDE2, PDE4, PDE5, and PDE7 (IC50s = 263, >300, 17.5, 49.1, and 58.3 μM, respectively). Milrinone (0.1-1 mg/kg) has positive inotropic effects, increasing cardiac contractile force in anesthetized dogs with a concomitant increase in heart rate but not blood pressure. It also increases contractile force in models of propranolol- and verapamil-induced heart failure in anesthetized dogs when administered at an initial dose of 30 μg/kg followed by a continuous 3 μg/kg per minute infusion. Milrinone has vasodilatory effects as well, decreasing mean aortic pressure and increasing venous compliance in anesthetized dogs when administered at an initial dose of 10 μg/kg followed by a continuous 1.7-2.4 μg/kg per minute infusion. Formulations containing milrinone have been used in the treatment of heart failure.
Chemical properties
Crystalline Solid
Originator
Eastman Kodak (Sterling) (USA)
The Uses of Milrinone
It is used (particularly intravenously, as the lactate) for the short-term management of severe heart failure. Milrinone inhibits the action of phosphodiesterase-3 preventing degradation of cAMP. The corresponding increase in cAMP levels leads to increased activation of protein kinase A. It is a selective phosphodiesterase inhibitor with vasodilating and positive inotropic activity.
The Uses of Milrinone
Selective phosphodiesterase inhibitor with vasodilating and positive inotropic activity. Cardiotonic
The Uses of Milrinone
antidepressant, 5HT reuptake inhibitor
The Uses of Milrinone
bronchodilator
Background
Heart failure is a multifactorial condition that affects roughly 1-2% of the adult population. Often the result of long-term myocardial ischemia, cardiomyopathy, or other cardiac insults, heart failure results from an inability of the heart to perfuse peripheral tissues with sufficient oxygen and metabolites, resulting in complex systemic pathologies. Heart failure is underpinned by numerous physiological changes, including alteration in β-adrenergic signalling and cyclic adenosine monophosphate (cAMP) production, which affects the heart's contractile function and cardiac output. Milrinone is a second-generation bipyridine phosphodiesterase (PDE) inhibitor created through chemical modification of amrinone. As a PDE-III inhibitor, milrinone results in increased cAMP levels and improves cardiac function and peripheral vasodilation in acute decongested heart failure.
Milrinone was originally synthesized at the Sterling Winthrop Research Institute in the 1980s. It was approved by the FDA on December 31, 1987, and was marketed under the trademark PRIMACOR? by Sanofi-Aventis US before being discontinued.
Indications
Milrinone is indicated for the short-term (48 hours or less) treatment of patients with acute decompensated heart failure. Milrinone administration should occur together with close monitoring using appropriate electrocardiographic equipment and should occur in a facility equipped for the immediate treatment of potential cardiac events, including ventricular arrhythmias.
What are the applications of Application
Milrinone is a phosphodietsterase 3 inhibitor
Definition
ChEBI: Milrinone is a member of the class of bipyridines that is 2-pyridone which is substituted at positions 3, 5, and 6 by cyano, pyrid-4-yl, and methyl groups, respectively. It is used (particularly intravenously, as the lactate) for the short-term management of severe heart failure. It has a role as an EC 3.1.4.17 (3',5'-cyclic-nucleotide phosphodiesterase) inhibitor, a platelet aggregation inhibitor, a vasodilator agent and a cardiotonic drug. It is a pyridone, a nitrile and a member of bipyridines.
Manufacturing Process
A mixture containing 20 g of 1-(4-pyridinyl)-2-propanone and 30 ml ofhexamethylphosphoramide was diluted with 65 ml of dimethylformamidedimethyl acetal and the resulting mixture was refluxed for 30 min. TLCanalysis showed a single spot, thereby indicating completion of the reaction(in another run, the reaction appeared to be complete after 30 min at roomtemperature). The mixture was evaporated under reduced pressure and apressure, thereby resulting in a crystalline residue weighing 24 g. The residuewas purified by continuous chromatographic extraction on alumina (about 150g) using refluxing chloroform as eluant. After 90 min, the extract was heatedin vacuo to remove the chloroform, thereby leaving, as a light yellowcrystalline material, 23.2 g of 1-(4-pyridinyl)-2-(dimethylamino)ethenylmethyl ketone, alternatively named 4-dimethylamino-2-(4-pyridinyl)-3-buten-2-one.
To a mixture containing 23 g of 1-(4-pyridinyl)-2-(dimethylamino)ethenylmethyl ketone and 11 g of α-cyanoacetamide dissolved in 400 ml ofdimethylformamide was added with stirring 14 g of sodium methoxide and theresulting reaction mixture was heated in an oil bath under gentle reflux forone hour. TLC analysis showed no starting material in the reaction mixturewhich was then concentrated in vacuo on a rotary evaporator to a volume ofabout 80 ml. The concentrate was treated with about 160 ml of acetonitrileand the resulting mixture was stirred on a rotary evaporator with warminguntil homogenous and then cooled. The crystalline product was collected,rinsed successively with acetonitrile and ether, and dried overnight at 55°C toyield 28 g of crystalline product, namely, sodium salt of 1,2-dihydro-6-methyl-2-oxo-5-(4-pyridinyl)nicotinonitrile, the presence of cyano being confirmed byIR analysis. An 8 g portion of said sodium salt was dissolved in 75 ml of hotwater, the aqueous solution treated with decolorizing charcoal, filtered, thefiltrate again treated with decolorizing charcoal and filtered, and the filtrateacidified with 6 N hydrochloric acid by dropwise addition to a pH of 3. Theacidic mixture was diluted with ethanol and cooled. The crystalline productwas collected, dried, recrystallized from dimethylformamide-water and dried toproduce 3.75 g of 1,2-dihydro-6-methyl-2-oxo-5-(4-pyridinyl)nicotinonitrile,m.p. >300°C.
Another method of preparation of 1,2-dihydro-6-methyl-2-oxo-5-(4-pyridinyl)nicotinonitrile (Patent US 4,413,127)
A 69.5 g portion of 1-ethoxy-2-(4-pyridinyl)ethenyl methyl ketone wasdissolved in 300 ml of ethanol and to the solution was added 13.2 g ofmalononitrile. The resulting mixture was refluxed for 5 hours, crystals startingto separate after about 30 min of refluxing. The reaction mixture was allowedto cool to room temperature and, the precipitate of fine needles was filtered,washed with ethanol and dried in a vacuum at 90°C to yield 25.4 g of 1,2-dihydro-6-methyl-2-oxo-5-(4-pyridinyl)-nicotinonitrile, m.p. >300°C.Concentration of the mother liquor provided another 2.1 g of product, m.p.>300°C.
To a aqueous solution of 1,2-dihydro-6-methyl-2-oxo-5-(4-pyridinyl)nicotinonitrile was added one molar equivalent of lactic acid to prepare themonolactate of 1,2-dihydro-6-methyl-2-oxo-5-(4-pyridinyl)nicotinonitrile.
brand name
Primacor (Sterling Winthrop);Corotrope.
Therapeutic Function
Cardiotonic
General Description
Milrinone, 1,6-dihydro-2-methyl-6-oxo-3,4'-bipyridine-5-carbonitrile (Primacor), is another dipyridinephosphodiesterase F-III inhibitor that possesses pharmacologicalproperties similar to those of amrinone. The inhibitionof the degradation of cAMP results in an increase in the cardiacmuscle’s force of contraction.
Biological Activity
Potent cAMP phosphodiesterase inhibitor (IC 50 = 56 nM for inhibition of PDE III). Has inotropic and vasodilator effects following oral or intravenous administration in vivo . Also available as part of the Phosphodiesterase Inhibitor Tocriset™ .
Biochem/physiol Actions
Phosphodiesterase type III inhibitor; cAMP-specific, cGMP-inhibitable; potent cardiotonic, positive inotropic vasodilator.
Pharmacokinetics
Milrinone is a bipyridine derivative with positive inotropic and lusitropic effects that also results in peripheral vasodilation with minimal chronotropic effects over a therapeutic range of 100 to 300 ng/mL. As such, milrinone is used in decompensated congestive heart failure. Studies have demonstrated that milrinone exhibits sigmoidal effects, such that increasing milrinone plasma concentrations beyond a certain level results in no further hemodynamic changes. Despite milrinone's benefits, both intravenous and oral use has been associated with increased frequency of ventricular arrhythmias, and long-term oral use has been associated with an increased risk of sudden death; in general, there are no data to support the safety or efficacy of milrinone use beyond 48 hours and patients should be monitored closely for cardiac dysfunction. Also, as milrinone is primarily excreted renally, dose adjustments may be required in patients with impaired renal function.
Metabolism
Animal studies suggest that two oxidative pathways are involved in milrinone metabolism, albeit only involving a small proportion of the administered dose. The major metabolite is the O-glucuronide metabolite.
Storage
Store at +4°C
References
[1] tang km1, jang ek, haslam rj. photoaffinity labelling of cyclic gmp-inhibited phosphodiesterase (pde iii) in human and rat platelets and rat tissues: effects of phosphodiesterase inhibitors. eur j pharmacol. 1994 jun 15;268(1):105-14.
[2] cheung p1, yang g, boden g. milrinone, a selective phosphodiesterase 3 inhibitor, stimulates lipolysis, endogenous glucose production, and insulin secretion. metabolism. 2003 nov;52(11):1496-500.
Properties of Milrinone
| Melting point: | >3000C |
| Boiling point: | 350.9°C (rough estimate) |
| Density | 1.1922 (rough estimate) |
| refractive index | 1.5700 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO: >10 mg/mL |
| form | powder |
| pka | 7.21±0.10(Predicted) |
| color | off-white |
| Water Solubility | Soluble in DMSO. Insoluble in water. |
| Merck | 14,6197 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 78415-72-2(CAS DataBase Reference) |
Safety information for Milrinone
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for Milrinone
| InChIKey | FYEJSUDMDUIMKJ-UHFFFAOYSA-N |
Milrinone manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 78415-72-2 Milrinone 98%View Details
78415-72-2 Milrinone 98%View Details
78415-72-2 -
 78415-72-2 Milrinone 99%View Details
78415-72-2 Milrinone 99%View Details
78415-72-2 -
 Milrinone CAS 78415-72-2View Details
Milrinone CAS 78415-72-2View Details
78415-72-2 -
 Milrinone, ≥98% CAS 78415-72-2View Details
Milrinone, ≥98% CAS 78415-72-2View Details
78415-72-2 -
 Milrinone 95% CAS 78415-72-2View Details
Milrinone 95% CAS 78415-72-2View Details
78415-72-2 -
 Milrinone CAS 78415-72-2View Details
Milrinone CAS 78415-72-2View Details
78415-72-2 -
 Milrinone CAS 78415-72-2View Details
Milrinone CAS 78415-72-2View Details
78415-72-2 -
 Milrinone CAS 78415-72-2View Details
Milrinone CAS 78415-72-2View Details
78415-72-2
