Gemcitabine hydrochloride
Synonym(s):2′-Deoxy-2′,2′-difluorocytidine; dFdC;dFdC;dFdCyd;Gemzar (Lilly);
- CAS NO.:122111-03-9
- Empirical Formula: C9H11F2N3O4.ClH
- Molecular Weight: 299.66
- MDL number: MFCD01735988
- EINECS: 601-823-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
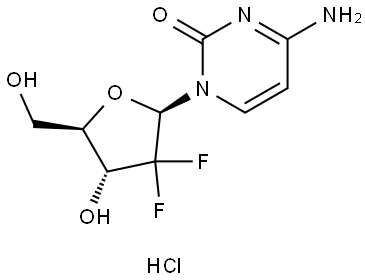
What is Gemcitabine hydrochloride?
Description
Gemcitabine is a novel nucleoside analog that was launched in 1995 in the Netherlands for the treatment of non-small cell lung cancer (nsclc) and in Sweden for pancreatic cancer. Gemcitabine is a prodrug which is phosphorylated intracellularly by deoxycytadine kinase to its active forms, the di- and triphosphates which bind to DNA competitively. This insertion inhibits processes required for DNA synthesis and metabolism, the essential function for both cell replication and repair. Furthermore, gemcitabine displays an extraordinary array of self-potentiating mechanisms that increase the concentration and prolong the retention of its active nucleotides in tumor cells. The title compound has shown activity against a wide spectrum of human solid tumors including colon, mammary, breast, bladder cancers. Synergistic activity of gemcitabine with other anticancer agents such as cisplatin has been reported.
Chemical properties
White crystalline granular, odorless
Originator
Lilly (U.S.A.)
The Uses of Gemcitabine hydrochloride
An antineoplastic
The Uses of Gemcitabine hydrochloride
An antineoplastic.
What are the applications of Application
Gemcitabine Hydrochloride is a deoxycytidine analog that inhibits ribonucleotide reductase & induces apoptosis
Definition
ChEBI: A 2'-deoxycytidine hydrochloriode having geminal fluoro substituents in the 2'-position. An inhibitor of ribonucleotide reductase, gemcitabine hydrochloride is used in the treatment of various carcinomas, including non-small cell lung cancer, pancreatic ca cer, bladder cancer and breast cancer.
brand name
Gemzar (Lilly).
Biological Activity
Deoxycytidine analog that inhibits DNA synthesis. Metabolized to form gemcitabine triphosphate (dFdCTP) and gemcitabine diphosphate (dFdCDP). dFdCTD inhibits ribonucleotide reductase causing a reduction in cellular nucleotides. dFdCTP is incorporated in DNA resulting in DNA strand termination. Displays antitumor activity in vitro and in vivo .
Biochem/physiol Actions
Gemcitabine is a widely used antitumor agents in both clinics and research labs. It is an antineoplastic agent and antimetabolite.
Veterinary Drugs and Treatments
Very limited clinical use and research performed with this drug to
date have demonstrated limited clinical efficacy. However, it potentially
may be useful as a radiosensitizer for non-resectable tumors,
as part of combination protocols, or as a single agent for tumors
not amenable to more accepted therapies. Follow research reports
for the most up-to-date information.
In humans, gemcitabine has shown some efficacy in treating
pancreatic carcinoma, small-cell lung carcinoma, lymphoma, bladder
and other soft tissue carcinomas.
storage
+4°C
References
1) Mini et al. (2006), Cellular Pharmacology of Gemcitabine; Ann. Oncol. 17 v7 2) Heinemann et al. (1995), Gemcitabine: a modulator of intracellular nucleotide and deoxynucleotide metabolism; Semin. Oncol. 22 11 3) Heinemann et al. (1992), Cellular elimination of 2′,2′-difluorodeoxycytidine 5’triphosphate: a mechanism of self-potentiation; Cancer Res. 52 533 4) Pourquier et al. (2002), Gemcitabine (2′,2′-difluoro-2′-deoxycytidine), an antimetabolite that poisons topoisomerase I; Clin.Cancer Res. 8 2499
Properties of Gemcitabine hydrochloride
| Melting point: | >250°C dec. |
| alpha | D +48°; 365 +257.9° (c = 1.0 in deuterated water) |
| storage temp. | 2-8°C |
| solubility | H2O: ≥10mg/mL |
| form | White powder |
| color | White |
| λmax | 268nm(H2O)(lit.) |
| Merck | 14,4386 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20° for up to 1 month. |
| CAS DataBase Reference | 122111-03-9(CAS DataBase Reference) |
Safety information for Gemcitabine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Gemcitabine hydrochloride
Gemcitabine hydrochloride manufacturer
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran

You may like
-
 Gemcitabine Hydrochloride 98%View Details
Gemcitabine Hydrochloride 98%View Details
122111-03-9 -
 122111-03-9 98%View Details
122111-03-9 98%View Details
122111-03-9 -
 Gemcitabine hydrochloride, ≥98% (HPLC) CAS 122111-03-9View Details
Gemcitabine hydrochloride, ≥98% (HPLC) CAS 122111-03-9View Details
122111-03-9 -
 Gemcitabine Hydrochloride CAS 122111-03-9View Details
Gemcitabine Hydrochloride CAS 122111-03-9View Details
122111-03-9 -
 122111-03-9 99%View Details
122111-03-9 99%View Details
122111-03-9 -
 122111-03-9 98%View Details
122111-03-9 98%View Details
122111-03-9 -
 Gemcitabine HCl 98% CAS 122111-03-9View Details
Gemcitabine HCl 98% CAS 122111-03-9View Details
122111-03-9 -
 Gemcitabine hydrochloride CAS 122111-03-9View Details
Gemcitabine hydrochloride CAS 122111-03-9View Details
122111-03-9
