Fexofenadine
- CAS NO.:83799-24-0
- Empirical Formula: C32H39NO4
- Molecular Weight: 501.66
- MDL number: MFCD00871892
- EINECS: 801-893-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
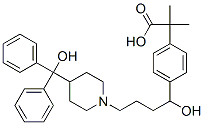
What is Fexofenadine?
Absorption
Fexofenadine is rapidly absorbed following oral administration and its absolute bioavailability is approximately 33%. The Tmax following oral administration is approximately 1-3 hours. The steady-state AUCss(0-12h) and Cmax following twice daily dosing of 60mg are 1367 ng/mL.h and 299 ng/mL, respectively.
Fexofenadine AUC is decreased by >20% when coadministered with fruit juices (e.g. apple, orange, grapefruit) due to their inhibition of OATP transporters - for this reason, prescribing information recommends administering fexofenadine only with water. Similarly, coadministration of fexofenadine with a high-fat meal appears to decrease AUC and Cmax by >20%.
Toxicity
No deaths were observed following the oral administration of up to 5000 mg/kg in both mice and rats (equivalent to approximately 100-200x the recommended human dose). Single doses of up to 800 mg and chronic exposure of up to 690 mg twice daily for 1 month in humans did not result in clinically significant adverse events. Symptoms of overdosage are consistent with fexofenadine's adverse effect profile and are likely to include dizziness, drowsiness, and dry mouth.
If overdosage occurs, employ symptomatic and supportive treatment. Hemodialysis does not effectively remove fexofenadine from the blood and is therefore of no benefit.
Description
Fexofenadine, the carboxylic acid metabolite of terfenadine, is widely available. It accounts for the antihistaminic properties of terfenadine, which is very rapidly metabolized via CYP3A4-catalyzed processes. Members of the organic anion transporter protein family and the drug efflux transporter P-glycoprotein are involved in the disposition of fexofenadine. Fexofenadine does not have the antiarrhythmic side effects of terfenadine.
Chemical properties
White Powder
Originator
Alernex,Dabur Pharmaceuticals Ltd.,India
The Uses of Fexofenadine
The active metabolite of Terfenadine (T114500), a H1-histamine receptor antagonist.
Background
Fexofenadine is an over-the-counter second-generation antihistamine used in the treatment of various allergic symptoms. It is selective for the H1 receptor, carries little-to-no activity at off-targets, and does not cross the blood-brain barrier - this is in contrast to previous first-generation antihistamines, such as diphenhydramine, which readily bind to off-targets that contribute to side effects such as sedation. Fexofenadine is the major active metabolite of terfenadine and is administered as a racemic mixture in which both enantiomers display approximately equivalent antihistamine activity.
Indications
In the United States, fexofenadine is indicated for the symptomatic treatment of allergic rhinitis in patients ≥2 years old and chronic idiopathic urticaria in patients ≥6 months old. In Canada, fexofenadine carries the same indications but is approved only for patients ≥12 years old. Fexofenadine is also available in combination with pseudoephedrine for the symptomatic treatment of season allergic rhinitis in patients ≥12 years old.
Definition
ChEBI: A piperidine-based anti-histamine compound.
Manufacturing Process
Synthesis of Fexofenadine (Patent U.S. No. 5,578,610
Aluminum chloride (44 g; 0.33 mol) was added in portions to a solution of
freshly distilled 4-chlorobutyryl chloride (17 mL; 0.15 mol) in 460 mL of
carbon disulfide at -10°C under a nitrogen atmosphere. The mixture was
stirred for 15 min, then the cooling bath was removed and the mixture was
allowed to warm to ambient temperature. The mixture was stirred then for 15
min more, then cooled again to -10°C and a solution of ethyl-α,α-
dimethylphenyl acetate (26.6 g; 0.14 mol) in 70 mL of carbon disulfide was
added dropwise. The mixture was stirred for 3 hours, then stirred overnight at
room temperature. The reaction mixture was partitioned between water and
CHCl3. The combined organic portions were dried over MgSO4, filtered and
concentrated in vacuo. The residue was dissolved in CH2Cl2 and filtered
through a plug of SiO2, eluting with 10% EtOAc in hexane. Yield of ethyl 3-
and 4-(4-chloro-1-oxobutyl)-α,α-dimethylphenylacetate 39.4 g (as a mixture
of aromatic regioisomers).
To a solution of 39.4 g of ethyl 3- and 4-(4-chloro-1-oxobutyl)-α,α-
dimethylphenylacetate dissolved in 800 mL of methanol and 200 mL of water
was added 40 g of NaOH. The mixture was refluxed for one hour. The cooled
mixture was then concentrated in vacuo. The concentrate was diluted with
water and washed with 2 portions of EtOAc. The aqueous layer was acidified
with concentrated HCl and extracted with 2 portions of EtOAc. The extracts
were dried over MgSO4, filtered, and concentrated in vacuo to afford 30.3 g of
crude product. The crude product was dissolved in 600 mL of EtOAc, 38 g of
cinchonidine was added, and the mixture was stirred overnight. The resulting
solids were filtered and washed with EtOAc and sucked dry under a rubber
dam to afford 25 g of a solid 4-(cyclopropyl-oxo-methyl)-α,α-
dimethylphenylacetic acid.
A solution of 10.5 g of 4-(cyclopropyl-oxo-methyl)-α,α-dimethylphenylacetic
acid in 250 mL of CH2Cl2 was cooled in an ice-MeOH bath and 25 g of
trimethylsilyliodide was then added via pipette. The mixture was stirred in the
ice bath for one hour, warmed to ambient temperature, and stirred for one
hour. A solution of aqueous sodium bisulfite was then added and the mixture
was stirred well. The phases were partitioned and the aqueous layer was
extracted with CH2Cl2. The combined organics were washed with saturated
aqueous NaCl, dried over MgSO4, filtered, and concentrated in vacuo to afford
12.6 g (77%) of 4-(4-iodo-1-oxobutyl)-α,α-dimethylphenylacetic acid.
To a solution of 12.6 g of 4-(4-iodo-1-oxobutyl)-α,α-dimethylphenylacetic acid
in 100 mL of ether cooled in an ice bath, was added 40 mL of ethereal CH2N2.
The mixture was stirred at 0°C for few minutes, then let stand for 2 hours. A
few drops of AcOH were added to decompose excess CH2N2, then the mixture
was filtered and stripped to afford 12.6 g (96%) of methyl 4-(4-iodo-1-
oxobutyl)-α,α-dimethylphenylacetate.
A solution of 12.6 g of methyl 4-(4-iodo-1-oxobutyl)-α,α-
dimethylphenylacetate in 500 mL of toluene in a one liter three neck flask was
added 8.8 g of 4-(α,α-diphenyl)piperidinemethanol and 23 g of K2CO3 and the
mixture was refluxed for 7 hours. The cooled reaction mixture was then
filtered and concentrated in vacuo. The residue was dissolved in ether and
treated with excess ethereal HCl. The mixture was then concentrated to a
solid. The solid was treated with EtOAc and collected by filtration. The product
was then partitioned between EtOAc and 2 N Na2CO3. The organics were dried
over MgSO4, filtered, and concentrated in vacuo to afford 13.5 g (79%) of
methyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-oxobutyl]-α,α-
dimethylphenylacetate.
A solution of 13.5 g of methyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-
1-oxobutyl]-α,α-dimethylphenylacetate in 250 mL of methanol was cooled in
an ice-methanol bath and 1.8 g of NaBH4 was added in portions. After 1 hour,
the mixture was concentrated to a so
Therapeutic Function
Antihistaminic
Pharmacokinetics
Fexofenadine relieves allergy symptoms by antagonizing the actions of histamine, an endogenous compound predominantly responsible for allergic symptomatology. The relatively long duration of action of fexofenadine (approximately 24 hours) allows for once or twice daily dosing, and its rapid absorption allows for an onset of action within 1-3 hours. Fexofenadine should not be taken with fruit juice, as this may impair its absorption.
Metabolism
Fexofenadine is very minimally metabolized, with only 5% of an ingested dose undergoing hepatic metabolism. The only identified metabolites are a methyl ester of fexofenadine (3.6% of the total dose) and MDL 4829 (1.5% of the total dose). The enzymes responsible for this metabolism have not been elucidated.
Properties of Fexofenadine
| Melting point: | 218-220°C |
| Boiling point: | 697.3±55.0 °C(Predicted) |
| Density | 1.171±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Methanol (Slightly, Heated, Sonicated) |
| form | Solid |
| pka | 4.43±0.10(Predicted) |
| color | White to Off-White |
| CAS DataBase Reference | 83799-24-0(CAS DataBase Reference) |
Safety information for Fexofenadine
Computed Descriptors for Fexofenadine
Fexofenadine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Fexofenadine HCL- IP/EP/USP 98%View Details
Fexofenadine HCL- IP/EP/USP 98%View Details
83799-24-0 -
 83799-24-0 Fexofinadine HCL 98%View Details
83799-24-0 Fexofinadine HCL 98%View Details
83799-24-0 -
 Fexofenadine 83799-24-0 98%View Details
Fexofenadine 83799-24-0 98%View Details
83799-24-0 -
 Fexofenadine 98%View Details
Fexofenadine 98%View Details -
 Fexofenadine 83799-24-0 99%View Details
Fexofenadine 83799-24-0 99%View Details
83799-24-0 -
 Fexofenadine 83799-24-0 98%View Details
Fexofenadine 83799-24-0 98%View Details
83799-24-0 -
 83799-24-0 Fexofenadine 99%View Details
83799-24-0 Fexofenadine 99%View Details
83799-24-0 -
 Fexofenadine 98% CAS 83799-24-0View Details
Fexofenadine 98% CAS 83799-24-0View Details
83799-24-0
