Terfenadine
Synonym(s):α-[4-(1,1-Dimethylethyl)phenyl]-4-(hydroxydiphenylmethyl)-1-piperidinebutanol
- CAS NO.:50679-08-8
- Empirical Formula: C32H41NO2
- Molecular Weight: 471.67
- MDL number: MFCD00079622
- EINECS: 256-710-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
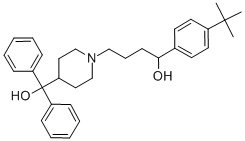
What is Terfenadine?
Absorption
On the basis of a mass balance study using 14C labeled terfenadine the oral absorption of terfenadine was estimated to be at least 70%
Toxicity
Mild (e.g., headache, nausea, confusion), but adverse cardiac events including cardiac arrest, ventricular arrhythmias including torsades de pointes and QT prolongation have been reported. LD50=mg/kg (orally in mice)
Chemical properties
White Solid
Originator
Histafen,Berk
The Uses of Terfenadine
Terfenadine has been used to study the role of histamine in itch related to proteinase-activated receptors (PARs) in mice. Terfenadine has also been used to block histamine receptor type 1 to study the pathogenesis of 2,4-dinitrobenzene sulfonic acid (DNBS)-induced ulcerative colitis in rats.
The Uses of Terfenadine
Nonsedating-type histamine H1-receptor antagonist. Antihistaminic
The Uses of Terfenadine
H1 antihistamine
The Uses of Terfenadine
It is used for relieving symptoms associated with seasonal allergic rhinitis and conjunctivitis, for angioneurotic edema and allergic skin reaction, and also as an ingredient of complex therapy for bronchial asthma. Synonyms of this drug are seldane, hystadin, trexil, and others.
Background
In the U.S., Terfenadine was superseded by fexofenadine in the 1990s due to the risk of cardiac arrhythmia caused by QT interval prolongation.
Indications
For the treatment of allergic rhinitis, hay fever, and allergic skin disorders.
What are the applications of Application
Terfenadine is a histamine H1-receptor antagonist, which blocks HERG and KIR6 channels
Definition
ChEBI: Terfenadine is a diarylmethane.
Manufacturing Process
A mixture of 107 g (0.4 mole) of α,α-diphenyl-4-piperidinemethanol, 105 g
(0.44 mole) of 4'-tert-butyl-4-chlorobutyrophenone, 70 g (0.7 mole) of
potassium bicarbonate, and a small amount of potassium iodide in 600 ml of
toluene was refluxed and stirred for 2.5 days then filtered. The filtrate was
treated with charcoal, filtered through celite then treated with ethereal HCl.
The resulting solid was recrystallized from methanol and isopropyl alcohol to
give the 4'-tert-butyl-4-[4-(α-hydroxy-α-phenylbenzyl)piperidino]-
butyrophenone hydrochloride, melting point 234°-235°C.
To a mixture of 4.2 g (0.0083 mole) of 4'-tert-butyl-4-[4-(α-hydroxy-α-
phenylbenzyl)piperidino]-butyrophenone hydrochloride and 0.54 g (0.01 mole)
of sodium methoxide in 25 ml of methanol is added 2.16 g (0.04 mole) of
potassium borohydride. The reaction mixture is stirred overnight, diluted with
water and the methanol removed under reduced pressure. The remaining
material is extracted with chloroform, washed with water, dried over
magnesium sulfate and filtered. The filtrate is concentrated, and the residue is
recrystallized from acetone-water to give 4-[α-(p-tert-butylphenyl)-α-
hydroxybenzyl]-α-phenyl-1-piperidinebutanol, melting point 161°-163°C.
brand name
Antifen;Fenadin.
Therapeutic Function
Antihistaminic, Bronchodilator
World Health Organization (WHO)
The first clinically interesting histamine H-receptor1 antagonists were introduced in the late 1940s and early 1950s. Several H-antihistaminics have a similar cardiac effect to that seen with astemizole1 and terfenadine. Serious cardiovascular adverse reactions have been reported when used concomitantly with imidazole antifungals and macrolide antibiotics. See also under astemizole.
Biological Activity
Histamine H 1 receptor antagonist. Also blocks hERG and K ATP channels (IC 50 values are 204 nM and 1.2 μ M respectively). Inhibits the delayed rectifier K + current (I Kr ) in guinea pig ventricular myocytes (IC 50 = 50 nM). Activity prolongs QT and induces Torsades de pointes (TdP); cardiotoxic in vivo .
Biochem/physiol Actions
Non-sedating second generation H1 histamine receptor antagonist. Mainly metabolized by Cyp3A4, 5, 7. Inhibits CYP2C8.
Pharmacokinetics
Terfenadine, an H1-receptor antagonist antihistamine, is similar in structure to astemizole and haloperidol, a butyrophenone antipsychotic. The active metabolite of terfenadine is fexofenadine.
Pharmacology
Terfenadine not only differs from the other antihistamine drugs in its chemical structure, but also in that its action begins within 1–2 h and last approximately 12 h, reaching its peak of action in 3–4 h.
Synthesis
Terfenadine, |á-(4-tert-butylphenyl) -4-hydroxydiphenylmethyl)- 1-piperidinebutanol (16.1.24), is synthesized in two ways. According to the first, benzyl-4- magnesiumchloropiperidine is reacted with benzophenone, giving (1-benzyl-4-piperidyl) diphenylcarbinol (16.2.22), which undergoes further debenzylation by reduction with hydrogen using a palladium over carbon catalyst, giving (4-piperidyl)diphenylcarbinol (16.2.22). This product is alkylated by either 1-(4-tert-butylphenyl)-4-chlorobutanol, which forms terfenadine (16.1.24), or by alkylating with (4-tert-butylphenyl-3-chloropropiophenone, which forms the product (16.1.25), the carbonyl group of which is reduced to an alcohol group, thus giving the desired terfenadine (16.1.24).
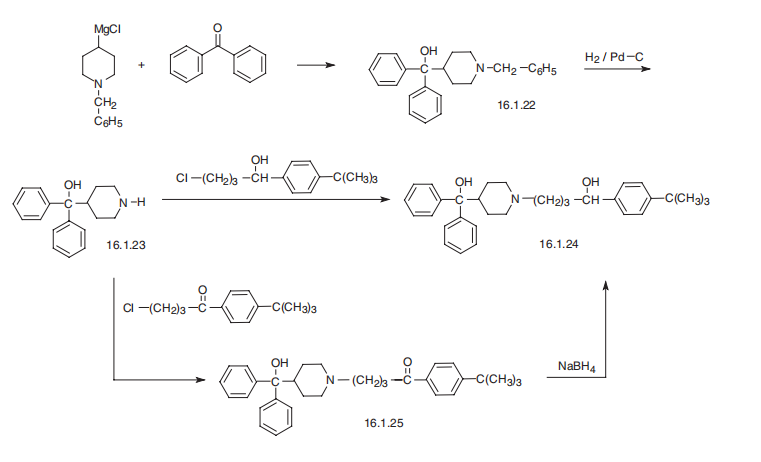
Metabolism
Hepatic
storage
Store at +4°C
Properties of Terfenadine
| Melting point: | 145-152 °C |
| Boiling point: | 572.76°C (rough estimate) |
| Density | 1.0488 (rough estimate) |
| refractive index | 1.6310 (estimate) |
| storage temp. | 2-8°C |
| solubility | chloroform: soluble250 mg plus 5 ml of solvent, clear to very slightly hazy, colorless to faintly yellow |
| form | neat |
| pka | pKa 9.21(H2O
t = 25
I = 0.025) (Uncertain) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | 0.001 g/100 mL (30 ºC) |
| CAS DataBase Reference | 50679-08-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Seldane(50679-08-8) |
Safety information for Terfenadine
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
Computed Descriptors for Terfenadine
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Terfenadine CAS 50679-08-8View Details
Terfenadine CAS 50679-08-8View Details
50679-08-8 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1