Favipiravir
Synonym(s):6-fluoro-3,4-dihydro-3-oxo-2-pyrazinecarboxamide;6-Fluoro-3-hydroxy-2-pyrazinecarboxamide;Avigan;T705;T-705
- CAS NO.:259793-96-9
- Empirical Formula: C5H4FN3O2
- Molecular Weight: 157.1
- MDL number: MFCD12032148
- EINECS: 1533716-785-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-24 18:06:02
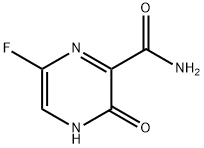
What is Favipiravir?
Absorption
The bioavailability of favipiravir is almost complete at 97.6%. The mean Cmax for the recommended dosing schedule of favipiravir is 51.5 ug/mL.
Studies comparing the pharmacokinetic effects of multiple doses of favipiravir in healthy American and Japanese subjects are below:
Japanese subjects First Dose:
Cmax = 36.24 ug/mL
tmax = 0.5 hr
AUC = 91.40 ugxhr/mL
American subjects First Dose:
Cmax = 22.01 ug/mL
tmax = 0.5 hr
AUC = 44.11 ugxhr/mL
Japanese Subjects Final Dose:
Cmax = 36.23 ug/mL
Tmax = 0.5 hr
AUC = 215.05 ugxhr/mL
American Subjects Final Dose:
Cmax = 23.94 ug/mL
Tmax = 0.6 hr
AUC = 73.27 ugxhr/mL
When favipiravir was given as a single dose of 400 mg with food, the Cmax decreased. It appears that when favipiravir is given at a higher dose or in multiple doses, irreversible inhibition of aldehyde oxidase (AO) occurs and the effect of food on the Cmax is lessened.
Toxicity
Based on single-dose toxicity studies, the lethal dose for oral and intravenous favipiravir in mice is estimated to be >2000 mg/kg. In rats, the lethal dose for oral administration is >2000 mg/kg, while the lethal dose in dogs and monkeys is >1000 mg/kg. Symptoms of overdose appear to include but are not limited to reduced body weight, vomiting, and decreased locomotor activity.
In repeat-dose toxicity studies involving dogs, rats, and monkeys, notable findings after administration of oral favipiravir included: adverse effects on hematopoietic tissues such as decreased red blood cell (RBC) production, and increases in liver function parameters such as aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT) and total bilirubin, and increased vacuolization in hepatocytes. Testis toxicity was also noted.
Favipiravir is known to be teratogenic; therefore, administration of favipiravir should be avoided in women if pregnancy is confirmed or suspected.
Toxicity information regarding favipiravir in humans is not readily available.
Description
Favipiravir, 6-fluoro-3-hydroxypyrazine-2-carboxamide, is a new broad-spectrum antiviral drug targeting RNA-dependent RNA polymerase (RdRp) developed by Japan's Toyama Chemical Pharmaceutical Company. It was approved for marketing in Japan in March 2014 for the treatment of new and recurrent influenza. During the outbreak of the new coronavirus, the results of the Phase I clinical study of the drug published in March 2020 showed that the drug may have the effect of speeding up virus clearance to alleviate the progress of new coronavirus pneumonia.
History
Favipiravir was originally developed in the late 1990s by a company that was later purchased by the Japanese firm Fujifilm as part of its transition from the photo business to healthcare. After being tested against a range of viruses, the drug was approved in Japan in 2014 for emergency use against flu epidemics or to treat new strains of influenza.
The Uses of Favipiravir
Favipiravir is used for the treatment of advanced Ebola virus infection in a small animal model. It suppressed the replication of Zaire Ebola virus and prevented a lethal outcome in 100% of the animals. Based on the studies, Favipiravir can be a candidate for the treatment of Ebola hemorrhagic fever. Favipiravir can be used for antiviral treatment of influenza A and B. Studies have shown that in addition to influenza virus, the drug also exhibits good antiviral activity against a variety of RNA viruses, such as Ebola virus, arena virus, Bunia virus, rabies virus and now COVID-19.
Background
Discovered by Toyama Chemical Co., Ltd. in Japan, favipiravir is a modified pyrazine analog that was initially approved for therapeutic use in resistant cases of influenza. This drug was developed to combat a disease other than EVD. The antiviral targets RNA-dependent RNA polymerase (RdRp) enzymes, which are necessary for the transcription and replication of viral genomes. Not only does favipiravir inhibit the replication of influenza A and B, but the drug has shown promise in the treatment of avian influenza. It may be an alternative option for influenza strains resistant to neuraminidase inhibitors. Favipiravir has been investigated for treating life-threatening pathogens such as Ebola virus, Lassa virus, and now COVID-19.
Indications
In 2014, favipiravir was approved in Japan to treat cases of influenza that were unresponsive to conventional treatment. Given its efficacy at targetting several strains of influenza, it has been investigated in other countries to treat novel viruses including Ebola and most recently, COVID-19.
Definition
ChEBI: Favipiravir is a member of the class of pyrazines that is pyrazine substituted by aminocarbonyl, hydroxy and fluoro groups at positions 2, 3 and 6, respectively. It is an anti-viral agent that inhibits RNA-dependent RNA polymerase of several RNA viruses and is approved for the treatment of influenza in Japan. It has a role as an antiviral drug, an anticoronaviral agent and an EC 2.7.7.48 (RNA-directed RNA polymerase) inhibitor. It is a primary carboxamide, a hydroxypyrazine and an organofluorine compound.
Mechanism of action
Favipiravir is an antiviral drug that selectively inhibited the RdRP of influenza virus. It showed specific activity against all three influenza A, B, and C (Furuta et al., 2013). It also inhibited the RV replication in HeLa cells, with an EC50 of 29 μg/mL (Furuta et al., 2002). Analysis showed that the primary mechanism of action of favipiravir against the influenza virus was specific inhibition of vRNA polymerase (Furuta et al., 2005). It is predicted that a similar mechanism might occur with other viruses, such as PV and RV, inhibited by favipiravir, which may account for its broad-spectrum inhibition. Mechanistic studies show that the favipiravir and its form favipiravir-RMP (favipiravir-ribofuranosyl-50-monophosphate) do not inhibit influenza RNA polymerase activity, but it is the phosphoribosylated form, favipiravir-ribofuranosyl-50-triphosphate (RTP) that inhibits the enzyme.
Pharmacokinetics
Favipiravir undergoes metabolic activation through ribosylation and phosphorylation to form the activated metabolite favipiravir-RTP in the tissues. Favipiravir at 1600 mg on day 1, then 400 mg twice-a-day from day 2 to day 6 followed by 400 mg once-a-day on day 7 has an estimated AUC of 1452.73 μg h/mL on day 1 and 1324.09 μg h/mL on day 7. It is primarily metabolized by the hepatic enzyme aldehyde oxidase and partially by xanthine oxidase to an inactive oxidative metabolite that is excreted in the hydroxylated form by the kidneys. It exhibits a nonlinear pharmacokinetics. Elimination half-life is 2–5.5 h, Bioavailability is 97.6%, mean Cmax is 51.5 μg/mL, parent volume of distribution is 15–20 L, and metabolites are cleared renally.
Synthesis
Favipiravir was synthesized in only six steps from 3-aminopyrazine-2-carboxylic acid with an overall yield of about 22.3%. Key intermediates 3 and 6 were obtained in excellent purity via recrystallization from optimized solvents, which was beneficial to large-scale production. In the key synthetic reaction, 3,6-dichloropyrazine-2-carbonitrile (6) was reacted sequentially, in one pot, with KF and 30% H2O2 to give (after crystallization from 95% EtOH) favipiravir as colorless crystals, with a 60% yield for this final step of the synthesis. (DOI:10.1007/s11696-017-0208-6)
645 grams of sodium hydroxide were dissolved in 9L of water, the temperature was lowered to 5??C, and 1.29 kg of 6-fluoro-3-hydroxy-2-cyanopyrazine was added in batches, stirred, and heated slightly, and the temperature of the reaction system was controlled to -10??C, it takes 3.5 hours to complete the addition, after holding for 1 hour, the temperature is raised to 40??C for 1 hour. Add 100g of activated carbon to the reaction solution, hot filter, cool the mother liquor to 5??C, adjust the pH to 3-4 with concentrated hydrochloric acid, precipitate a large amount of solids, filter and dry to obtain a crude off-white powder, beaten with 2.8 liters of 15% methanol aqueous solution and filter After drying, 1.34 kg of white powder favipiravir was obtained. 1H-NMR (DMSO, 600MHz): |? 13.38 (s, 1H), 8.73 (1s, 1H), 8.51-8.49 (d, J=12, 2H) (yield 91%).
Mechanisms of action
Favipiravir inhibited the replication of the viral genome, which was the most manifested in the middle of the viral proliferation cycle in a time-of-drug-addition test. The anti-viral activity of favipiravir was attenuated in the presence of purine nucleosides or purine bases, indicating competition of favipiravir with purine nucleosides rather than pyrimidine nucleosides. Favipiravir exerts its anti-viral activity as a pro-drug since favipiravir is intra-cellularly phosphoribosylated to be an active form, favipiravir-RTP, which inhibits the viral replication by interacting with viral RNA polymerase. The mechanism of the interaction of favipiravir-RTP with the RdRp molecule has not been fully elucidated. It is hypothesized that favipiravir may be misincorporated in a nascent viral RNA or act by binding to conserved polymerase domains, thus preventing the incorporation of nucleotides for viral RNA replication and transcription. Favipiravir-RTP binds to and inhibits RNA-dependent RNA polymerase (RdRp), which ultimately prevents viral transcription and replication.
Metabolism
Favipiravir is extensively metabolized with metabolites excreted mainly in the urine. The antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to the inactive metabolite, T705M1.
storage
Store at -20°C
References
1) Furuta?et al.?(2002),?In Vitro and In Vivo Activities of Anti-Influenza Virus Compound T-705; Antimicrob. Agents Chemother.,?46?977 2) Takahashi?et al.?(2003)?In Vitro and In Vivo Activities of T-705 and Oseltamivir Against Influenza Virus; Antivir. Chem. Chemother.,?14?235 3) Sleeman?et al.?(2010)?In Vitro Antiviral Activity of Favipiravir (T-705) against Drug-Resistant Influenza and 2009 A(H1N1) Viruses; Antimicrob. Agents Chemother.,?54?2517 4) Furuta?et al.?(2005)?Mechanism of action of T-705 against influenza virus; Antimicrob. Agents Chemother.,?49?981 5) Furuta?et al.?(2013)?), Favipiravir (T-705), a Novel Viral RNA Polymerase Inhibitor; Antiviral Res.,?100?446 6) Dong?et al.?(2020)?Discovering Drugs to Treat Coronavirus Disease 2019 (COVID-19); Drug Discov. Ther.,?14?58 7) Tu?et al.?(2020)?A Review of SARS-CoV-2 and the Ongoing Clinical Trials; Int. J. Mol. Sci., 21?E2657
Properties of Favipiravir
| Melting point: | >151°C (dec.) |
| Density | 1.78±0.1 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | Soluble in DMSO (30 mg/mL), water (12 mg/mL with warming) |
| form | solid |
| pka | 8.77±0.60(Predicted) |
| color | Off-white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or water may be stored at -20° for up to 3 months. |
Safety information for Favipiravir
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Favipiravir
Favipiravir manufacturer
TAGOOR LABORATORIES PVT LTD
Inchem Laboratories Pvt Ltd
Optimus Pharma Pvt Ltd (Sekhmet Pharmaventures))
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 3-Nitrobenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 FAVIPIRAVIR 98%View Details
FAVIPIRAVIR 98%View Details
259793-96-9 -
 Favipiravir 99%View Details
Favipiravir 99%View Details -
 Favipiravir 98%View Details
Favipiravir 98%View Details
259793-96-9 -
 Favipiravir 99%View Details
Favipiravir 99%View Details -
 Favipiravir 259793-96-9 98%View Details
Favipiravir 259793-96-9 98%View Details
259793-96-9 -
 Favipiravir 98%View Details
Favipiravir 98%View Details -
 FAVIPIRAVIR 95-99 %View Details
FAVIPIRAVIR 95-99 %View Details
259793-96-9 -
 Favipiravir CAS 259793-96-9View Details
Favipiravir CAS 259793-96-9View Details
259793-96-9