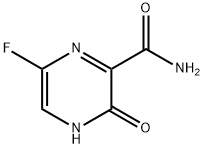
259793-96-9
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
PRODUCT INTRODUCTION
Description
Favipiravir, an oral, broad-spectrum RdRp inhibitor, is an already approved drug for new and reemerging pandemic influenza in Japan and has an established and well-characterized safety profile. It was discovered by chemical modification of a pyrazine analog initially screened by in vitro anti-influenza virus activity in cells. Favipiravir is a selective and potent influenza viral RNA polymerase inhibitor effective against all subtypes and strains of influenza viruses, including ones sensitive or resistant to marketed neuraminidase and M2 inhibitors. Favipiravir demonstrated anti-viral activities against other RNA viruses. Hence, favipiravir is a promising drug for treating infections caused not only by influenza viruses but also by a wide range of RNA viruses. On the other hand, favipiravir has a risk for teratogenicity and embryotoxicity.