Oseltamivir
- CAS NO.:196618-13-0
- Empirical Formula: C16H28N2O4
- Molecular Weight: 312.4
- MDL number: MFCD00953939
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-24 14:33:46
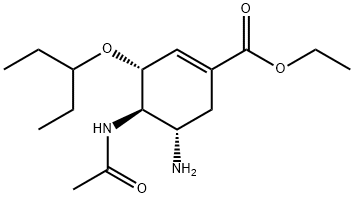
What is Oseltamivir?
Absorption
Oseltamivir is readily absorbed from the gastrointestinal tract after oral administration of oseltamivir phosphate and is extensively converted by predominantly hepatic esterases to the active metabolite oseltamivir carboxylate. At least 75 % of an oral dose reaches the systemic circulation as the active metabolite. Exposure to the pro-drug is less than 5 % relative to the active metabolite. Plasma concentrations of both pro-drug and active metabolite are proportional to dose and are unaffected by co-administration with food. Pharmacokinetic parameters following twice daily dosing of oseltamivir 75mg capsules are as follows: Cmax of oseltamivir and oseltamivir carboxylate were found to be 65ng/mL and 348ng/mL, respectively, while AUC (0-12h) of oseltamivir and oseltamivir carboxylate were found to be 112ng·h/mL and 2719ng·h/mL, respectively.
Toxicity
Reports of overdoses with oseltamivir have been received from clinical trials and during postmarketing experience. In the majority of cases reporting overdose, no adverse reactions were reported. Adverse reactions reported following overdose were similar in nature to those observed with therapeutic doses of oseltamivir.
Originator
Tamiflu,Hoffmann-La Roche Inc
The Uses of Oseltamivir
Oseltamivir is an orally active inhibitor of influenza virus neuraminidase; converted in vivo to the active acid metabolite. An antiviral drug. It is a COVID19-related research product.
Indications
According to FDA prescribing information, oseltamivir is indicated for the treatment of acute, uncomplicated illness due to influenza A and B infection in patients 2 weeks of age and older who have been symptomatic for no more than 48 hours . In particular, this agent is indicated in adults and children including full-term neonates who present with symptoms typical of influenza when influenza virus is circulating in the community. Efficacy has been demonstrated when treatment is initiated within two days of the first onset of symptoms.
Oseltamivir is also indicated for the prophylaxis of influenza in patients one year and older . Specifically, post-exposure prevention in individuals one year of age or older following contact with a clinically diagnosed influenza case when influenza virus is circulating in the community qualifies for such prophylactic therapy. Oseltamivir would only be indicated for post-exposure prevention of influenza in infants less than 1 year of age during a pandemic influenza outbreak.
Background
Oseltamivir (marketed as the product Tamiflu?), is an antiviral neuraminidase inhibitor used for the treatment and prophylaxis of infection with influenza viruses A (including pandemic H1N1) and B. Oseltamivir exerts its antiviral activity by inhibiting the activity of the viral neuraminidase enzyme found on the surface of the virus, which prevents budding from the host cell, viral replication, and infectivity.
The clinical benefit of use of oseltamivir is greatest when administered within 48 hours of the onset of influenza symptoms since effectiveness decreases significantly after that point in time; there is generally no benefit in use beyond 48 hours for healthy, low-risk individuals as influenza is a self-limiting illness. However, antiviral treatment might be beneficial when initiated after 48 hours for patients with severe, complicated or progressive illness or for hospitalized patients. According to the CDC, data from clinical trials and observational studies have demonstrated that early antiviral treatment can shorten the duration of fever and illness symptoms, and may reduce the risk of some complications (including pneumonia and respiratory failure). They recommend the use of oseltamivir in people with a higher risk of developing complications including children younger than 2 years, people over 65 years, people with some chronic conditions or immunosuppression, pregnant women, residents of long term care facilities, and indigenous communities for example.
The benefits of oseltamivir use are controversial; a 2014 Cochrane Review of the evidence found that oseltamivir treatment had limited benefit. The authors concluded that oseltamivir use in healthy adults had small, non-specific effects on symptoms (where the time to first alleviation of symptoms was only reduced from 7 to 6.3 days), it had no effect on hospitalizations, and that there was no evidence for any reductions in complications of influenza such as pneumonia. Due to the risk of adverse effects such as nausea, vomiting, psychiatric effects and renal adverse events in adults and vomiting in children, the harms are generally considered to outweigh the small clinical benefit of use of oseltamivir.
Notably, in 2017, the World Health Organization downgraded oseltamivir from its essential medicines list from a "core" drug to a "complementary" drug, due to limited cost-effectiveness. Yearly vaccination with the influenza vaccine is still considered the best preventative measure.
Definition
ChEBI: A cyclohexenecarboxylate ester that is the ethyl ester of oseltamivir acid. An antiviral prodrug (it is hydrolysed to the active free carboxylic acid in the liver), it is used to slow the spread of influenza.
Indications
Oseltamivir phosphate (Tamiflu) is the ethyl ester prodrug of oseltamivir carboxylate, an analogue of neuraminic (sialic) acid that is a reversible competitive antagonist of influenza A and B neuraminidase.Influenza virus resistant to oseltamivir has not been found in naturally acquired isolates but has been isolated from influenza patients who have undergone treatment with this drug.These resistant strains contain mutations in the active site of neuraminidase and are generally less virulent and infective than nonresistant virus. In vitro passage of influenza virus in the presence of oseltamivir carboxylate can produce mutations in hemagglutinin that decrease the overall dependence of viral replication on neuraminidase; however, the clinical relevance of this resistance mechanism is unknown.
Therapeutic Function
Antiviral
Antimicrobial activity
Oseltamivir is active against influenza A and B, but no other virus.
Acquired resistance
Mutations in the neuraminidase (H274Y) have been detected in treated patients with seasonal H1N1 infection. Cross-resistance with zanamivir has been described in vitro.
Pharmaceutical Applications
A selective neuraminidase inhibitor, formulated as the phosphate salt of the ethyl ester for oral administration.
Pharmacokinetics
There have been postmarketing reports of delirium and abnormal behavior leading to injury, and in some cases resulting in fatal outcomes, in patients with influenza who were receiving oseltamivir. Because these events were reported voluntarily during clinical practice, estimates of frequency cannot be made but they appear to be uncommon based on oseltamivir. These events were reported primarily among pediatric patients and often had an abrupt onset and rapid resolution. The contribution of oseltamivir to these events has not been established. Influenza can be associated with a variety of neurologic and behavioral symptoms that can include events such as hallucinations, delirium, and abnormal behavior, in some cases resulting in fatal outcomes. These events may occur in the setting of encephalitis or encephalopathy but can occur without obvious severe disease.
Pharmacokinetics
Oral absorption: c. 75%
Cmax 75 mg oral: 0.35–0.55 mg/L after 4 h
Plasma half-life: 7–9 h
Plasma protein binding: Not known
The ethyl ester prodrug is hydrolyzed by hepatic esterases
to release the active compound, oseltamivir carboxylate.
Drug is excreted in the urine as the carboxylate
derivative.
Clinical Use
Treatment and prevention of susceptible influenza A (H3N2) and B infections in adults and young children
Clinical Use
Oseltamivir is approved for the treatment of uncomplicated acute influenza in patients aged 1 year and older. It decreases the duration of illness by 1 to 1.5 days when treatment is initiated within 48 hours of the onset of symptoms. Oseltamivir is also indicated for the prophylaxis of influenza in individuals aged 13 and older. It reduces infection rates to approximately 10 to 25% of that found in untreated populations; however, it is not intended to substitute for the early vaccination recommended by the CDC. Oseltamivir can be used as postexposure prophylaxis in household contacts of infected patients, with infection rates of treated patients around 10% of placebo control levels.
Side Effects
Adverse events relate to the gastrointestinal tract; the most common is nausea with or without vomiting in 10% of patients. Food alleviates side effects.
Side Effects
The most frequently reported adverse effects of oseltamivir are nausea and vomiting.These events are usually mild to moderate, occur during the first 1 to 2 days of treatment, and can be lessened by taking the drug with food. Bronchitis, insomnia, and vertigo may also occur. Oseltamivir may not be indicated for use in certain individuals. Its efficacy in patients with chronic cardiac or respiratory disease has not been established. In clinical trials, no difference in the incidence of complications was seen between treatment and control groups. The efficacy of oseltamivir has not been demonstrated in immunocompromised patients, patients who begin treatment after 40 hours of symptoms, or patients given repeated prophylactic courses of therapy. Dosage adjustment is recommended for individuals with renal insufficiency; the drug’s safety in patients with hepatic insufficiency is unknown.
Metabolism
Oseltamivir is extensively converted to the active metabolite, oseltamivir carboxylate, by esterases located predominantly in the liver. Oseltamivir carboxylate is not further metabolized. Neither oseltamivir nor oseltamivir carboxylate is a substrate for, or inhibitor of, cytochrome P450 isoforms. No phase 2 conjugates of either compound have been identified in vivo.
Metabolism
Oseltamivir is a prodrug; it is extensively metabolised by
esterases in the liver to the active carboxylate metabolite.
Oseltamivir carboxylate is not further metabolised and
is eliminated entirely by renal excretion. Renal clearance
exceeds glomerular filtration rate indicating that tubular
secretion occurs in addition to glomerular filtration. Less
than 20% of an oral radiolabelled dose is eliminated in
faeces.
Properties of Oseltamivir
| Melting point: | 107-108 °C |
| Boiling point: | 473.3±45.0 °C(Predicted) |
| Density | 1.08±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C(protect from light) |
| solubility | Chloroform (Sparingly), DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Sli |
| form | Solid |
| pka | 7.7 (25°); 6.6 (70°) |
| color | Off-White to Pale Beige |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 196618-13-0 |
| EPA Substance Registry System | 1-Cyclohexene-1-carboxylic acid, 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-, ethyl ester, (3R,4R,5S)- (196618-13-0) |
Safety information for Oseltamivir
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Oseltamivir
Oseltamivir manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 196618-13-0 Oseltamivir 98%View Details
196618-13-0 Oseltamivir 98%View Details
196618-13-0 -
 Oseltamivir 98%View Details
Oseltamivir 98%View Details -
 Oseltamivir 99%View Details
Oseltamivir 99%View Details
196618-13-0 -
 Oseltamivir 196618-13-0 98%View Details
Oseltamivir 196618-13-0 98%View Details
196618-13-0 -
 196618-13-0 Oseltamivir 99%View Details
196618-13-0 Oseltamivir 99%View Details
196618-13-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
