Etravirine
- CAS NO.:269055-15-4
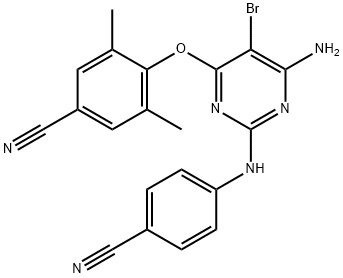
- Empirical Formula: C20H15BrN6O
- Molecular Weight: 435.28
- MDL number: MFCD09837879
- EINECS: 682-331-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
What is Etravirine?
Absorption
Maximum oral absorption is achieved in 2.5-4 hours.
Absorption is unaffected by the concomitant use of oral ranitidine or omeprazole, which decrease gastric acidity.
Administration under fasting conditions resulted in a near 50% decrease in systemic exposure (AUC) when compared to administration after a meal.
Description
Etravirine is a second-generation NNRTI. It is indicated for use in
combination with other antiretroviral agents for treating HIV-1 infection in
treatment-experienced adult patients who have evidence of viral replication and HIV-1 strains resistant to the currently available NNRTIs and
other antiretroviral agents. The NNRTIs, along with nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs/NtRTIs), are important
components of the combination regimens currently used to treat HIV-1
infection. The NRTIs/NtRTIs act by competing with the natural nucleotide
substrates of reverse transcriptase for incorporation into viral DNA
and subsequent chain termination. By contrast, the NNRTIs bind to an
allosteric site of the enzyme and disrupt the DNA polymerase function by
inducing conformational changes to the catalytic site. The allosteric
binding nature of NNRTIs generally results in improved safety profile
since there is no known human homolog for the drug-binding site of
the enzyme.
As with other NNRTIs, etravirine has
many drug–drug interactions. It is a substrate of CYP3A4, CYP2C9,
and CYP2C19, an inducer of 3A4, and an inhibitor of 2C9 and 2C19.
Caution should be used with co-administration of inducers, inhibitors, or
substrates of these enzymes. Etravirine can be synthesized starting from
5-bromo-2,4,6-trichloropyrimidine through three successive nucleophilic
substitution reactions. Initial displacement with 4-aminobenzonitrile using
Hu¨nig’s base, followed by reaction with sodium salt of 4-hydroxy-3,5-
dimethylbenzonitrile, and subsequent ammonolysis reaction with ammonia in dioxane under pressure affords etravirine.
.
Chemical properties
White to Off-White Solid
Originator
Janssen (United States)
The Uses of Etravirine
Etravirine is an antiretroviral (anti-HIV) drug that is part of the non nucleoside reverse transcriptase inhibitor (NNRTIs or Non Nukes) family. It is used together with other antiretrovirals in treatment-experienced adult patients, who have failed previous therapy, and have HIV-1 strains which are resistant to other retrovirals and NNRTIs. Etravirine is used to delay the progression of HIV infection. By using etravirine, your immune system should improve (increase in CD4 + count) and you will be better protected against opportunistic infections.
Etravirine does not cure AIDS or completely kill the HIV virus, but helps to prevent further damage by slowing down the production of new viruses. Treatment with etravirine does not reduce the risk of passing infection on to others. You will still be able to pass HIV by sexual contact, by blood transfer or by sharing needles. You should always use appropriate precautions to prevent passing HIV on to others.
The Uses of Etravirine
Etravirine (TMC125) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) used for the treatment of HIV.
The Uses of Etravirine
Etravirine is a novel HIV reverse transcriptase inhibitor useful in treatment of HIV infection.
Background
Etravirine is an antiretroviral agent more specifically classified as a Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI). Etraverine is used clinically for the treatment of human immunodeficiency virus type 1 (HIV-1) infection. On January 18, 2007, the FDA granted accelerated approved for the use of etravirine 100mg tablets in the treatment of adult HIV-1 infection documented to be resistant to therapy with other NNRTIs and antiretroviral agents. On March 26, 2012, approval was extended for use in treatment-experienced pediatric patients 6 to 18 years of age, weighing at least 16 kg. Etravarine must always be used in combination with other antiretroviral drugs.
Etravirine exerts its effects via direct inhibition of the reverse transcriptase enzyme of human immunodeficiency virus type 1 (HIV-1), and consequently blocks DNA-dependent and RNA-dependent polymerase activity. Etravirine does not inhibit human DNA polymerase alpha, beta or gamma.
Common side effects of use include mild to moderate rash within the first 6 weeks of therapy, nausea, diarrhea and peripheral neuropathy. Patients are advised to immediately contact their healthcare provider if a rash develops.
In 2009, postmarketing case reports of Stevens-Johnson Syndrome, toxic epidermal necrolysis, erythema multiforme, and other hypersensitivity reactions lead to a revision of etravirine's "Warnings and Precautions," as well as notification of health care providers.
In 2013, reports of Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) in the setting of immune reconstitution, as well as more in depth information about the development of rashes in patients taking etravirine, lead to a modification of etravirine's monograph.
Indications
Etravirine is indicated, in combination with other antiretroviral agents, for the treatment of HIV-1 infection in treatment-experienced patients ≥2 years of age.
What are the applications of Application
Etravirine is a novel HIV reverse transcriptase inhibitor
What are the applications of Application
Etravirine-d6 is a novel labeled HIV reverse transcriptase inhibitor
Definition
ChEBI: An aminopyrimidine that consists of 2,6-diaminopyrimidine bearing a bromo substituent at position 5, a 4-cyano-2,6-dimethylphenoxy substituent at position 4 and having a 4-cyanophenyl substituent attached to the 2-amino group. NNRTI of HIV-1, binds directl to RT and blocks RNA-dependent and DNA-dependent DNA polymerase activities
brand name
Intelence
Acquired resistance
Various mutations are associated with a decreased virological response. Single codon substitutions at positions 100, 101 and 181 are considered major mutations. A single K103N mutation is not associated with resistance.
Pharmaceutical Applications
A comprehensive analysis of baseline resistance data from the DUET-1 and DUET-2 studies has identified a list of 17 etravirine resistance associated mutations: V901, A98G, L100L, K101E/H/I, V1061, E138A, V179D/F/T, Y181C/L/V, G190A/S, and M230L. A single K103N mutation is not associated with resistance to etravirine.
Mechanism of action
Etravirine binds directly to reverse transcriptase and blocks the RNA-dependent and DNA-dependent DNA polymerase activities by causing a disruption of the enzyme's catalytic site. Etravirine does not inhibit the human DNA polymerases alpha, beta, and gamma.
Pharmacokinetics
Clinical trials have shown no prolongation of QT intervals on electrocardiograms after 8 days of dosing.
Pharmacokinetics
Oral absorption: Not known/available
Cmax 200 mg oral twice daily: c. 959 ng/mL
Cmin 200 mg oral twice daily: c. 469 ng/mL
Plasma half-life: c. 36 h
Volume of distribution: Not known/available
Plasma protein binding: >99%
Administration with food improves the bioavailability and
reduces interpatient variability. It undergoes oxidative metabolism
by cytochrome P450 systems. Around 93.7% and 1.2%
of an administered dose can be retrieved in the feces and
urine, respectively, mostly as unchanged drug.
Details of distribution into CSF, semen and breast milk
and recommendations for dose adjustment in patients with
hepatic impairment are not yet available.
Clinical Use
Treatment of HIV-1 infection in adults (in combination with other antiretroviral drugs)
Side Effects
In the phase III studies around 15% of patients experienced
erythematous or maculopapular rashes of mild or moderate
severity; most resolved with continued dosing, but treatment
was discontinued in 2% of patients. Rare cases of Stevens–
Johnson syndrome have been reported.
Other common adverse events are diarrhea, nausea, headache
and fatigue. Dyslipidemia and raised pancreatic amylase
occur in some patients.
Synthesis
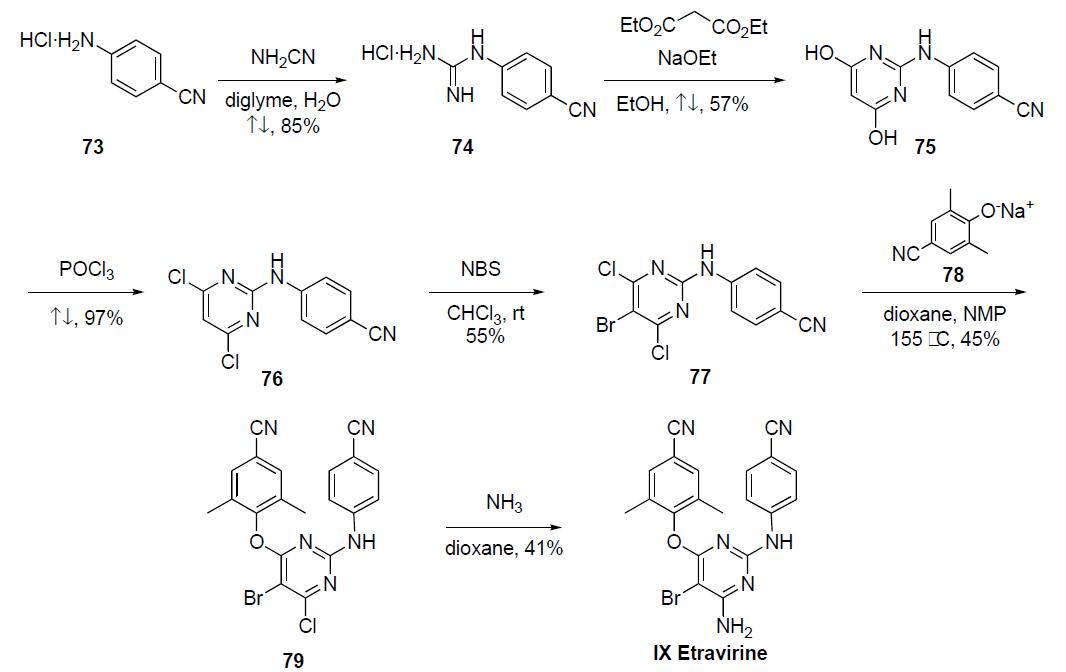
Only the discovery synthesis and small scale syntheses have been disclosed for this compound in the following scheme. The largest scale synthesis was initiated by the portionwise addition of cyanamide to a solution of the p-cyanoaniline hydrochloride salt 73. The mixture was refluxed in diglyme to give guanidine salt 74 in 85% yield after concentration of the reaction mixture and recrystallization from acetone. Reaction of guanidine 74 with diethylmalonate in the presence of sodium ethoxide in refluxing ethanol gave pyrimidine diol 75 in 57% yield, which upon refluxing in phosphorous oxychloride for 30 min gave dichloride 76 in 97% yield. Bromination of dichloride 76 with NBS in chloroform at room temperature provided bromide 77 in 55% yield. Heating a mixture of the dichlorobromide 77 with the sodium salt of 2,5-dimethyl-4-cyanophenol 78, generated by reaction with sodium hydride in situ) in diglyme and NMP at 155 ??C gave the coupled product 79 in 45% yield. Finally, reaction of the chloride 79 with ammonia in refluxing dioxane (or iPrOH) in a sealed tube gave etravirine (IX) in 41% yield after purification.
Drug interactions
Potentially hazardous interactions with other drugs Antibacterials: concentration increased by clarithromycin, also concentration of clarithromycin reduced; concentration of both drugs reduced with rifabutin; avoid concomitant use with rifampicin. Antivirals: concentration possibly reduced by efavirenz and nevirapine - avoid concomitant use; concentration of fosamprenavir increased, consider reducing fosamprenavir dose; possibly reduces bosutinib and indinavir concentration - avoid concomitant use; concentration of dolutegravir reduced; possibly reduces concentration of maraviroc; concentration reduced by tipranavir and tipranavir concentration increased - avoid concomitant use. Clopidogrel: possibly reduced antiplatelet effect. Guanfacine: possibly reduces concentration of guanfacine - increase guanfacine dose. Orlistat: absorption possibly reduced by orlistat.
Metabolism
Metabolized (in vitro) by the liver CYP450 enzymes: CYP3A4, CYP2C9, CYP2C19. The major metabolites formed by a methyl hydroxylation of the dimethylbenzonitrile moiety retained less than 90% of etravirine's activity.
Metabolism
Etravirine is extensively metabolised by hepatic microsomal enzymes, mainly by the cytochrome P450 isoenzymes CYP3A4, CYP2C9, and CYP2C19, to substantially less active metabolites.Unchanged etravirine accounted for 81.2-86.4% of the administered dose in faeces. Unchanged etravirine was not detected in urine.
Properties of Etravirine
| Melting point: | .265°C (dec.) |
| Boiling point: | 637.4±65.0 °C(Predicted) |
| Density | 1.439 g/cm3 |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated, Sonicated) |
| form | Solid |
| pka | 1.23±0.10(Predicted) |
| color | White to Off-White |
Safety information for Etravirine
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
Computed Descriptors for Etravirine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile](https://img.chemicalbook.in/CAS/GIF/500287-72-9.gif)

You may like
-
 269055-15-4 Etravirine 98%View Details
269055-15-4 Etravirine 98%View Details
269055-15-4 -
 269055-15-4 98%View Details
269055-15-4 98%View Details
269055-15-4 -
 Etravirine 99%View Details
Etravirine 99%View Details
269055-15-4 -
 Etravirine >98% (HPLC) CAS 269055-15-4View Details
Etravirine >98% (HPLC) CAS 269055-15-4View Details
269055-15-4 -
 Etravirine 98% (HPLC) CAS 269055-15-4View Details
Etravirine 98% (HPLC) CAS 269055-15-4View Details
269055-15-4 -
 Etravirine 95.00% CAS 269055-15-4View Details
Etravirine 95.00% CAS 269055-15-4View Details
269055-15-4 -
 4-((6-Amino-5-bromo-2-((4-cyanophenyl)amino)pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile CAS 269055-15-4View Details
4-((6-Amino-5-bromo-2-((4-cyanophenyl)amino)pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile CAS 269055-15-4View Details
269055-15-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
