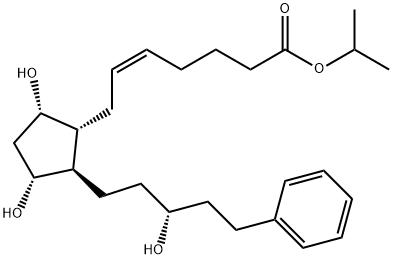
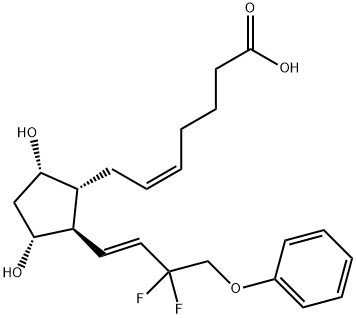
Tafluprost
- CAS NO.:209860-87-7
- Empirical Formula: C25H34F2O5
- Molecular Weight: 452.53
- MDL number: MFCD08062150
- EINECS: 234-199-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
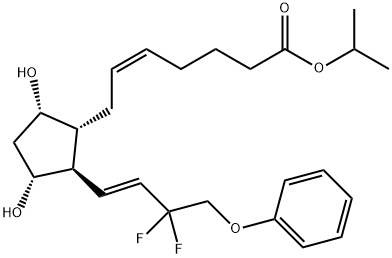
What is Tafluprost?
Absorption
Following instillation, tafluprost is absorbed through the cornea and is hydrolyzed to the biologically active acid metabolite, tafluprost acid. Tafluprost is an ester which makes the drug lipophillic enough to be quickly absorbed through. When administered to the eye, the peak plasma concentration (Cmax) and time to peak plasma concentration (Tmax) of tafluprost acid in healthy subjects was 26 pg/mL and 10 minutes respectively. a AUC, tafluprost acid = 394 pgmin/mL - 432 pgmin/mL.
Toxicity
Most common ocular adverse reaction is conjunctival hyperemia (range 4% – 20%).
Description
Glaucoma is second only to cataracts as a causative factor of blindness.
By 2010, it is estimated that approximately 60 million people worldwide
will be afflicted by glaucoma, so effective treatments should garner a large market.
PG analogs have been widely used for lowering IOP by increasing uveoscleral outflow through agonism of the prostanoid FP
receptor, and currently marketed versions include latanoprost, unoprostone isopropyl ester, bimatoprost, and travoprost.
Compared to the carboxylic acid of latanaprost
(Ki=4.7 nM), the carboxylic acid of tafluprost displayed a 10-fold greater
affinity for the prostanoid FP receptor (Ki=0.4 nM). The synthesis of
tafluprost begins with a Wittig condensation of the protected bicyclic
lactone carbaldehyde with a dimethyl phosphonate ketone derivative.
Compared to the carboxylic acid of latanaprost (Ki 4.7 nM), the carboxylic acid of tafluprost displayed a 10-fold greater affinity for the prostanoid FP receptor (Ki 0.4 nM). The synthesis of tafluprost begins with a Wittig condensation of the protected bicyclic lactone carbaldehyde with a dimethyl phosphonate ketone derivative. The bottom appendage is then completed by the fluorination of the ketone with morpholino-sulfur trifluoride. Hydrolysis of the benzoate ester protecting group liberates the hydroxy group, and reduction of the lactone is accomplished with aluminum hydride to generate the lactol. Condensation of this intermediate with the phosphonium salt of the acid side chain generates the free acid, or active ingredient, which is subsequently esterified with 2-iodopropane in the presence of DBU.
.
Originator
Santen/Asahi Glass (Japan)
The Uses of Tafluprost
Tafluprost is a novel prostanoid used in the treatment of glaucoma and is the first prostanoid to be released in a preservative free-formula.
The Uses of Tafluprost
Tafluprost is a new potent PGF2_ analogue for the treatment of glaucoma and a potent and selective FP receptor agonist. It lowers intraocular pressure (IOP) primarily by increasing uveoscleral outflow. Tafluprost has been shown to have a potent IOP-lowering effect on elevated IOP caused by open-angle glaucoma, or by diseases known as ocular hypertension.
Background
A prostaglandin analogue ester prodrug used topically (as eye drops) to control the progression of glaucoma and in the management of ocular hypertension. Chemically, tafluprost is a fluorinated analog of prostaglandin F2-alpha. Tafluprost was approved for use in the U.S. on February 10, 2012.
Indications
Tafluprost is indicated for reducing elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
What are the applications of Application
Tafluprost is an FP receptor agonist
Definition
ChEBI: Tafluprost is a prostaglandin Falpha that is prostaglandin F2alpha in which the carboxylic acid function has been converted to the corresponding isopropyl ester and the 3-hydroxy-1-octenyl side-chain is substituted by 3,3-difluoro-4-phenoxybut-1-enyl. Used for treatment of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. It has a role as a prostaglandin receptor agonist. It is a prostaglandins Falpha, an organofluorine compound and an isopropyl ester. It is functionally related to a prostaglandin F2alpha.
brand name
Taflotan
Pharmacokinetics
Tafluprost is a novel prostaglandin analog with a high affinity for the fluoroprostaglandin (FP) receptor PGF2α. Tafluprost has an affinity for the FP receptor that is approximately 12 times higher than that of the carboxylic acid of latanoprost, but with almost no potential to bind to other receptors.
Side Effects
Common side effects of Tafluprost Eye Drops include: stinging or burning sensation, redness, itching, dryness and irritation of the eyes, darkening of the skin of the eyelids and changes in the length/thickness/number/colour of eyelashes. Patients with mixed colour irises may also experience darkening of the iris. Severe allergic reactions are rare and present with symptoms such as swelling of the face/eyes/lips/tongue, difficulty breathing, and an itchy rash around the eyes or over the body. Seek medical attention if any of these serious adverse reactions occur.
Synthesis
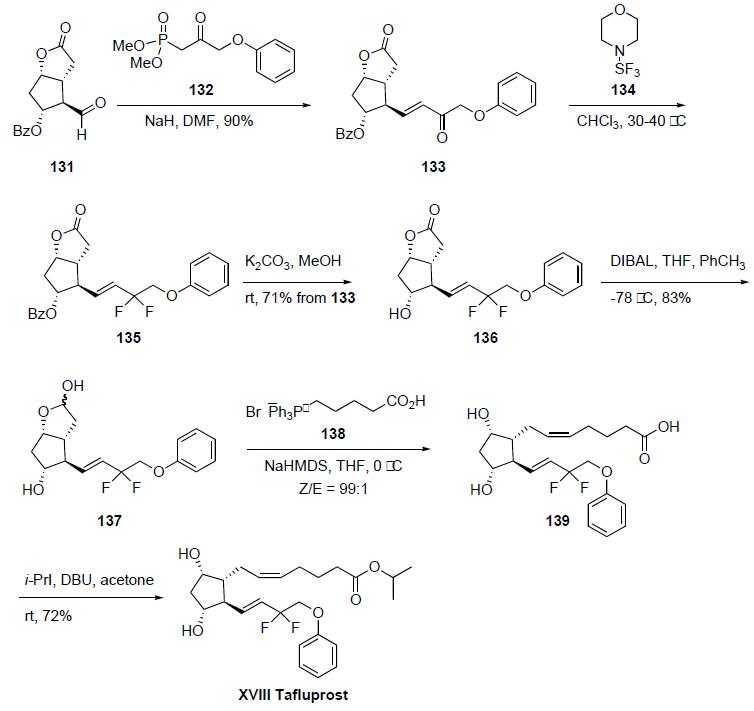
The synthesis was initiated from the Corey aldehyde 131. Horner-Emmons condensation of the bicyclic carbaldehyde 131 with the dimethyl phosphonate 132 using NaH in DMF gave enone 133 in 90% yield. Fluorination of the enone 133 was accomplished upon reaction with morpholino-sulfur trifluoride (134) in chloroform to yield the corresponding difluorinated compound 135. Hydrolysis of the benzoate ester group of 135 with K2CO3 in methanol at room temperature afforded alcohol 136 in 71% yield from 133. Reduction of the lactone group of 136 with diisobutyl aluminum hydride (DIBAL) in THF/toluene gave lactol 137 in 83% yield. Lactol 137 was condensed with the phosphonium ylide prepared by deprotonation of phosphonium salt 138 with sodium bis(trimethylsilyl)amide (NaHMDS) in THF/toluene to give the prostaglandin F2-alpha derivative 139 in excellent Z/E selectivity (99:1). The synthesis was completed by esterification of compound 139 with isopropyl iodide and DBU in acetone to provide tafluprost (XVIII) in 72% yield.
Metabolism
Tafluprost is an ester prodrug which is rapidly hydrolyzed by corneal esterases to form its biologically active acid metabolite. Tafluprost acid is further metabolized via fatty acid β-oxidation and phase II conjugation into 1,2,3,4-tetranor acid.
Mode of action
Tafluprost(209860-87-7) is a Prostaglandin Analog. The mechanism of action of tafluprost is as a Prostaglandin Receptor Agonist. It is believed that prostanoid FP-receptor agonists such as tafluprost reduce IOP by increasing the uveoscleral outflow of aqueous humor. There is some evidence that tafluprost may lower IOP by interaction with the EP3 receptor.
DB08819
Properties of Tafluprost
| Boiling point: | 552.9±50.0 °C(Predicted) |
| Density | 1.186 |
| storage temp. | 2-8°C |
| solubility | soluble in Chloroform, DMSO, Ethyl Acetate |
| pka | 14.48±0.70(Predicted) |
| form | Clear, colorless to slightly yellow oil |
| color | Colorless to light yellow |
Safety information for Tafluprost
Computed Descriptors for Tafluprost
| InChIKey | WSNODXPBBALQOF-VEJSHDCNSA-N |
| SMILES | C(OC(C)C)(=O)CCC/C=C\C[C@H]1[C@@H](O)C[C@@H](O)[C@@H]1/C=C/C(F)(F)COC1=CC=CC=C1 |
Tafluprost manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 209860-87-7 Tafluprost 98%View Details
209860-87-7 Tafluprost 98%View Details
209860-87-7 -
 209860-87-7 99%View Details
209860-87-7 99%View Details
209860-87-7 -
 209860-87-7 98%View Details
209860-87-7 98%View Details
209860-87-7 -
 209860-87-7 98%View Details
209860-87-7 98%View Details
209860-87-7 -
 Tafluprost CAS 209860-87-7View Details
Tafluprost CAS 209860-87-7View Details
209860-87-7 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
