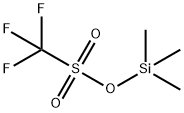
Ethyl trifluoromethanesulfonate
- CAS NO.:425-75-2
- Empirical Formula: C3H5F3O3S
- Molecular Weight: 178.13
- MDL number: MFCD00000410
- EINECS: 207-037-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
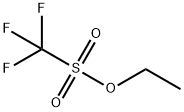
What is Ethyl trifluoromethanesulfonate ?
Description
Ethyl trifluoromethanesulfonate is a cationic polymerization agent used to produce polyurethane, polyacrylate, and other synthetic resins. It is an effective drug for the treatment of HIV infection and chronic bronchitis. Ethyl trifluoromethanesulfonate has been shown to inhibit the replication of HIV-1 virus at concentrations as low as 1 μM when tested in vitro. The mechanism of this drug's anti-HIV activity is unknown and may involve the inhibition of reverse transcriptase or proteases. Ethyl trifluoromethanesulfonate can be detected in vivo up to 4 hours after administration. This drug is metabolized into trifluoroacetic acid by esterases, glycosidases, and/or oxidases.
Chemical properties
Clear colorless to yellow liquid
The Uses of Ethyl trifluoromethanesulfonate
Ethyl trifluoromethanesulfonate is a powerful ethylating agent. due to the strong electron-absorbing ability of trifluoromethanesulfonyl, the reactivity is much higher than that of conventional alkylation reagents such as bichloride or alkyl sulfonate.
Synthesis
Triethyl orthoformate (4.44g,30mmol) was slowly added to trifluoromethanesulfonic anhydride (8.47g,30mmol) under ice bath, transferred to 25°C for reaction, monitored by NMR for 15 min, and the reaction was completed, distilled under reduced pressure to obtain 10.15g of ethyl trifluoromethanesulfonate colorless liquid in 94% yield.
Purification Methods
The ester reacts slowly with H2O and aqueous alkali. If its IR has no OH bands (~3000 cm-1) then purify it by redistillation. If OH bands are present, then dilute with dry Et2O and shake (carefully) with aqueous NaHCO3 until effervescence ceases, then wash with H2O and dry (MgSO4), filter, evaporate and distil the residue under a slight vacuum then at atmospheric pressure in a N2 atmosphere. IT IS A POWERFUL ALKYLATING AGENT, AND THE FUMES ARE VERY TOXIC — PERFORM ALL OPERATIONS IN AN EFFICIENT FUME CUPBOARD. [Gramstad & Haszeldine J Chem Soc 173 1956, Howells & McCown Chem Rev 77 69 1977, Beilstein 3 IV 34.]
Properties of Ethyl trifluoromethanesulfonate
| Boiling point: | 115 °C (lit.) |
| Density | 1.374 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 96 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Chloroform, Methanol (Slightly) |
| form | Oil |
| color | Colourless |
| Specific Gravity | 1.374 |
| Water Solubility | Hydrolyzes in water. |
| Sensitive | Hygroscopic |
| BRN | 1770746 |
| Stability: | Volatile |
| CAS DataBase Reference | 425-75-2(CAS DataBase Reference) |
| EPA Substance Registry System | Methanesulfonic acid, trifluoro-, ethyl ester (425-75-2) |
Safety information for Ethyl trifluoromethanesulfonate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H226:Flammable liquids H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ethyl trifluoromethanesulfonate
| InChIKey | UVECLJDRPFNRRQ-UHFFFAOYSA-N |
New Products
2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Strontium Carbonate, 98% Wang resin Sodium hydrogenphosphate, anhydrous 2-Bromo-3-methoxyaniline hydrochloride, 95% (Custom work) 1-Bromo-4-chlorobenzene, 99% Benzocaine, 98% (R)-2-Methylpyrolidine-2-carboxylic acid (De Mepro) Ramipril Sacubitril- Valsartan Boc-his(trt)-OH Fmoc-L-Glu-OtBu Boc-L-Tyr(tBu)-OH Semi carbazide Hydrochloride 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 2-Chloromethyl-4-methyl-quinazoline Trans-4-Aminocyclohexanol [4tac] 2-[1-(Mercaptomethyl)Cyclopropyl]Acetic AcidRelated products of tetrahydrofuran
You may like
-
 425-75-2 ETHYL TRIFLATE 98%View Details
425-75-2 ETHYL TRIFLATE 98%View Details
425-75-2 -
 425-75-2 98%View Details
425-75-2 98%View Details
425-75-2 -
 Ethyl trifluoromethanesulfonate CAS 425-75-2View Details
Ethyl trifluoromethanesulfonate CAS 425-75-2View Details
425-75-2 -
 Ethyl trifluoromethanesulphonate, 99% CAS 425-75-2View Details
Ethyl trifluoromethanesulphonate, 99% CAS 425-75-2View Details
425-75-2 -
 Ethyl Trifluoromethanesulfonate CAS 425-75-2View Details
Ethyl Trifluoromethanesulfonate CAS 425-75-2View Details
425-75-2 -
 Ethyl trifluoromethanesulfonate 98%View Details
Ethyl trifluoromethanesulfonate 98%View Details -
 Ethyl trifluoromethanesulfonate 95% CAS 425-75-2View Details
Ethyl trifluoromethanesulfonate 95% CAS 425-75-2View Details
425-75-2 -
 Ethyl trifluoromethanesulfonate CAS 425-75-2View Details
Ethyl trifluoromethanesulfonate CAS 425-75-2View Details
425-75-2
