Methyl trifluoromethanesulfonate
Synonym(s):Methyl triflate;Methyl trifluoromethanesulfonate;Trifluoromethanesulfonic acid methyl ester
- CAS NO.:333-27-7
- Empirical Formula: C2H3F3O3S
- Molecular Weight: 164.1
- MDL number: MFCD00000409
- EINECS: 206-371-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
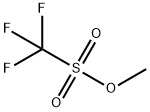
What is Methyl trifluoromethanesulfonate?
Chemical properties
clear colourless to yellow liquid
The Uses of Methyl trifluoromethanesulfonate
Methyl trifluoromethanesulfonate is used as a powerful methylating reagent in chemistry. Further, it is used in the conversion of amines to methyl ammonium triflates. In addition, it is also used in Suzuki reaction.
What are the applications of Application
Methyl trifluoromethanesulfonate is a product used as a methylation reagent
General Description
Methyl trifluoromethane sulfonate is a brown liquid. Insoluble in water. (NTP, 1992) This material is a very reactive methylating agent, also known as methyl triflate.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Methyl trifluoromethanesulfonate is sensitive to heat. . Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition Methyl trifluoromethanesulfonate emits toxic fumes.
Fire Hazard
Methyl trifluoromethanesulfonate is combustible.
Toxicology
Methyl trifluoromethanesulfonate is extremely hazardous. All possible precautions should be taken to avoid inhalation or absorption through the skin. This material is extremely destructive to the tissue of the mucous membranes and upper respiratory tract, eyes, and skin. Inhalation may be fatal as a result of spasm, inflammation, and edema of the larynx and bronchi, chemical pneumonitis, and pulmonary edema. Use in a fume hood. Although there has yet to be a reported human fatality, several cases were reported for methyl fluorosulfonate (LC50 (rat, 1 h) = 5 ppm), and methyl triflate is expected to have similar toxicity based on available evidence.
Purification Methods
Methyl trifluoromethanesulfonate (methyl triflate) [333-27-7] M 164.1, b 97 -97.5o/736mm, 9 9o/~760mm, 100-102o/~760mm, d 4 1.496, n D 1.3238. It is a strong methylating agent but is corrosive and POISONOUS. Fractionate it carefully and collecting the middle fraction (use an efficient fume cupboard) and keep away from moisture. It is a POWERFUL ALKYLATING AGENT and a strong IRRITANT. [IR: Gramstad & Haszeldine J Chem Soc 173 1956 , J Chem Soc 4069 1957 .] Trifluoromethanesulfonic acid (triflic acid) [1493-13-6] M 151.1, boils higher (b 162o/atm), has a pKa of 3.10, and is TOXIC and hygroscopic. [Hansen J Org Chem 30 4322 1965, Kurz & El-Nasr J Am Chem Soc 104 5823 1982, Beilstein 3 IV 34.]
Properties of Methyl trifluoromethanesulfonate
| Boiling point: | 94-99 °C(lit.) |
| Density | 1.45 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 101 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Sparingly), Ethyl Acetate (Slightly) |
| form | Liquid |
| color | Clear colorless to yellow |
| Specific Gravity | 1.450 |
| Sensitive | Air Sensitive |
| BRN | 774772 |
| Stability: | Hygroscopic, Moisture Sensitive, Volatile |
| InChI | InChI=1S/C2H3F3O3S/c1-8-9(6,7)2(3,4)5/h1H3 |
| CAS DataBase Reference | 333-27-7(CAS DataBase Reference) |
| EPA Substance Registry System | Methyl trifluoromethanesulfonate (333-27-7) |
Safety information for Methyl trifluoromethanesulfonate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H314:Skin corrosion/irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methyl trifluoromethanesulfonate
| InChIKey | OIRDBPQYVWXNSJ-UHFFFAOYSA-N |
| SMILES | C(F)(F)(F)S(OC)(=O)=O |
Methyl trifluoromethanesulfonate manufacturer
JSK Chemicals
New Products
(R)-1-Boc-3-hydroxypyrrolidine Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
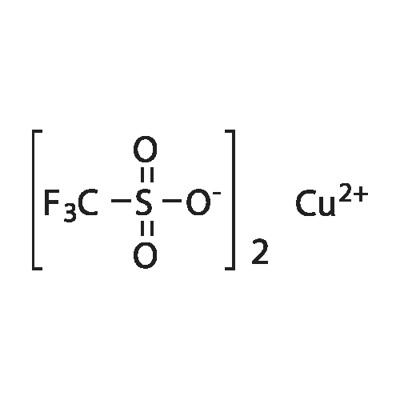
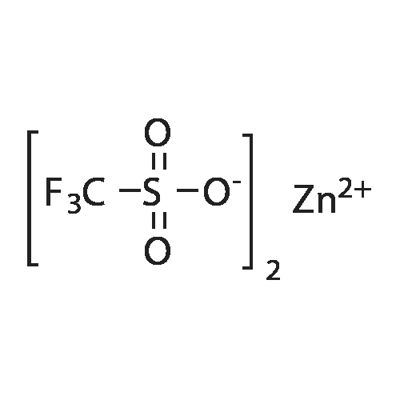
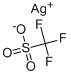

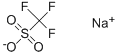
You may like
-
 METHYL TRIFLATE 98%View Details
METHYL TRIFLATE 98%View Details
333-27-7 -
 Methyl trifluoromethanesulfonate 333-27-7 98%View Details
Methyl trifluoromethanesulfonate 333-27-7 98%View Details
333-27-7 -
 Methyl trifluoromethanesulfonate 98% (GC) CAS 333-27-7View Details
Methyl trifluoromethanesulfonate 98% (GC) CAS 333-27-7View Details
333-27-7 -
 Methyl trifluoromethanesulfonate CAS 333-27-7View Details
Methyl trifluoromethanesulfonate CAS 333-27-7View Details
333-27-7 -
 Methyl Trifluoromethanesulfonate CAS 333-27-7View Details
Methyl Trifluoromethanesulfonate CAS 333-27-7View Details
333-27-7 -
 Methyl trifluoromethanesulfonate, 95% 99%View Details
Methyl trifluoromethanesulfonate, 95% 99%View Details
333-27-7 -
 Methyl trifluoromethanesulfonate CAS 333-27-7View Details
Methyl trifluoromethanesulfonate CAS 333-27-7View Details
333-27-7 -
 Methyl trifluoromethanesulfonate CAS 333-27-7View Details
Methyl trifluoromethanesulfonate CAS 333-27-7View Details
333-27-7
