Sodium trifluoromethanesulfonate
Synonym(s):Sodium triflate;Trifluoromethanesulfonic acid sodium salt
- CAS NO.:2926-30-9
- Empirical Formula: CF3NaO3S
- Molecular Weight: 172.06
- MDL number: MFCD00061607
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-12 18:21:52
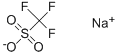
What is Sodium trifluoromethanesulfonate?
Description
Sodium trifluoromethanesulfonate is commonly known as trifluoromethanesulfonic acid or TFMS. Possessing unique properties, TFMS is water-soluble, anionic, and boasts a range of valuable attributes. As a colorless, odorless, and non-toxic substance, TFMS finds widespread use as a reagent in organic synthesis, a catalyst in industrial applications, and a buffer in various biochemical experiments. In scientific research, TFMS stands out due to its robust acidity, enabling it to act as a potent catalyst in organic synthesis. Additionally, it effectively serves as a reliable buffer at pH levels, ensuring precise pH maintenance in biochemical experiments. As an anionic compound, TFMS functions as a strong acid in solution, facilitating the donation of protons to other molecules, thus reducing the pH.
Chemical properties
White to off-white powder
The Uses of Sodium trifluoromethanesulfonate
Sodium trifluoromethanesulfonate is used in the preparation of N-fluoro-2-methylpyridinium triflate by reaction with dinitrogen difluoride as a reagent. It is also used as a chaotropic mobile phase additive in reversed-phase liquid chromatography (RP-LC).
The Uses of Sodium trifluoromethanesulfonate
Sodium trifluoromethanesulfonate can be employed as a reagent for the preparation of:
- Aryl fluorides via silver-catalyzed fluorination of arylstannanes.
- Ionic liquids such as N, N -dialkylpyrrolidinium triflate, N,N-dialkylimidazolium triflate, and N-alkylpyridinium triflate.
It can be also used as supporting electrolyte in electrochemical O-glycosylation of primary alcohols with O-protected thioglycosides.
Preparation
Sodium trifluoromethanesulfonate is prepared by adding a slight excess of sodium sulfate to an aqueous solution of barium trifluoromethanesulfonate. The reaction mixture is stirred for several minutes and allowed to stand for one day, and the white precipitate of barium sulfate is removed by filtration. The clear filtrate is evaporated to dryness, and the solid product is recrystallized from dry acetone. The salt is dried under vacuum at 110?°C.1 Barium trifluoromethanesulfonate can be easily prepared from aqueous trifluoromethanesulfonic acid and barium carbonate. Other methods include the preparation of sodium trifluoromethane sulfonate from sodium dithionite and bromotrifluoromethane and its oxidation to sodium trifluoromethane sulfonate using hydrogen peroxide.
General Description
Sodium trifluoromethanesulfonate (Sodium triflate or NaOTf) is an efficient catalyst as well as a reagent in many organic reactions. The prominent application includes catalytic asymmetric Mannich-type reactions, Mannich-type reactions in water, and Diels-Alder reactions.
Solubility in organics
soluble in water, alcohol, acetonitrile, N,N-dimethylformamide, and most highly polar organic solvents.
Properties of Sodium trifluoromethanesulfonate
| Melting point: | 253-255 °C (lit.) |
| storage temp. | Inert atmosphere,Room Temperature |
| form | Powder |
| color | White to off-white |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| BRN | 3728797 |
| CAS DataBase Reference | 2926-30-9(CAS DataBase Reference) |
Safety information for Sodium trifluoromethanesulfonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium trifluoromethanesulfonate
| InChIKey | KKVTYAVXTDIPAP-UHFFFAOYSA-M |
Sodium trifluoromethanesulfonate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Sodium trifluoromethanesulfonate CAS 2926-30-9View Details
Sodium trifluoromethanesulfonate CAS 2926-30-9View Details
2926-30-9 -
 Sodium trifluoromethanesulfonate CAS 2926-30-9View Details
Sodium trifluoromethanesulfonate CAS 2926-30-9View Details
2926-30-9 -
 Sodium trifluoromethanesulfonate CAS 2926-30-9View Details
Sodium trifluoromethanesulfonate CAS 2926-30-9View Details
2926-30-9 -
 Sodium trifluoromethanesulphonate, 98% CAS 2926-30-9View Details
Sodium trifluoromethanesulphonate, 98% CAS 2926-30-9View Details
2926-30-9 -
 Sodium Trifluoromethanesulfonate CAS 2926-30-9View Details
Sodium Trifluoromethanesulfonate CAS 2926-30-9View Details
2926-30-9 -
 Sodium trifluoromethanesulfonate 98.00% CAS 2926-30-9View Details
Sodium trifluoromethanesulfonate 98.00% CAS 2926-30-9View Details
2926-30-9 -
 Sodium trifluoromethanesulfonate 95% CAS 2926-30-9View Details
Sodium trifluoromethanesulfonate 95% CAS 2926-30-9View Details
2926-30-9 -
 Sodium trifluoromethanesulfonate CAS 2926-30-9View Details
Sodium trifluoromethanesulfonate CAS 2926-30-9View Details
2926-30-9
