Scandium trifluoromethanesulfonate
Synonym(s):Sc(OTf)3;Sc(OTf)3;Scandium tris(trifluoromethanesulfonate);Scandium(III) trifluoromethanesulfonate;Trifluoromethanesulfonic acid scandium(III) salt
- CAS NO.:144026-79-9
- Empirical Formula: C3F9O9S3Sc
- Molecular Weight: 492.16
- MDL number: MFCD00192433
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-12 18:45:48
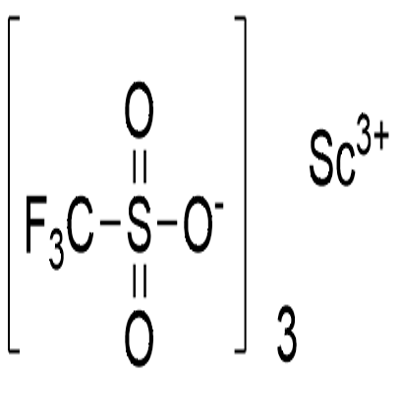
What is Scandium trifluoromethanesulfonate?
Description
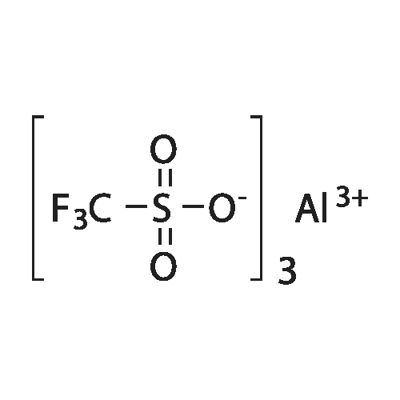
Scandium trifluoromethanesulfonate, commonly called Scandium(III) triflate, is a chemical compound with formula Sc(SO3CF3)3, a salt consisting of scandium cations Sc3+ and triflate SO3CF3? anions.
Scandium(III) triflate is an extremely active, efficient, recoverable and reusable acylation catalyst. Its an important catalyst for the Friedel-Crafts acylation, Diels-Alder reactions and other carbon-carbon bond-forming reactions. It also stereochemically catalyzes the radical polymerization of acrylates. Scandium(III) triflate complex of (4′S,5′S)-2,6-bis[4′-(triisopropylsilyl)oxymethyl-5′-phenyl-1′,3′-oxazolin-2′-yl]pyridine has been employed as catalyst for the asymmetric Friedel-Crafts reaction between substituted indoles and methyl (E)-2-oxo-4-aryl-3-butenoates.
Chemical properties
White powder
The Uses of Scandium trifluoromethanesulfonate
Scandium(III) trifluoromethanesulfonate is widely used as a catalyst in hydrothiolation, selective two-electron reduction of oxygen by ferrocene derivatives and vinylogous Fridel-crafts alkylation of indoles and pyrrole in water. It is involved in the Mukaiyama aldol addition and stereochemically catalyzes the radical polymerization of acrylates. It acts as a Lewis acid catalyst and used in the synthesis of bullvalone via a stabilized sulfur ylide.
The Uses of Scandium trifluoromethanesulfonate
Scandium Triflate is an important catalyst used in Friedel-Crafts acylation, Baylis-Hillman reaction and other carbon-carbon bond forming reactions.
What are the applications of Application
Scandium(III) triflate is an efficient acylation catalyst
What are the applications of Application
Scandium(III) triflate was used as a catalyst in:
Hydrothiolation reaction of aromatic and aliphatic thiols.
Selective two-electron reduction of O2 by ferrocene derivatives.
Vinylogous Friedel-Crafts alkylation of indoles and pyrroles in water.
Synthesis of β-cyanoketones.
Combination with triethylsilane to reductively open functionalized pyranoside rings.
The key steps of synthesis of bullvalone via a stabilized sulfur ylide.
Reactions
- Water tolerant Lewis acid.
- Commonly used in a range of Lewis acid catalyzed reactions.
- Efficient metal source for Lewis acid catalyzed asymmetric reactions.
- Catalyzes Friedel-Crafts alkylation, acylation and related reactions.
- Catalyzes various domino- and multi-component processes.
- Catalyzes electrophilic additions of alpha-diazoesters with ketones.
- Catalyzes carbon insertion reactions.



General Description
Scandium(III) triflate is an extremely active, efficient, recoverable and reusable acylation catalyst. Its an important catalyst for the Friedel-Crafts acylation, Diels-Alder reactions and other carbon-carbon bond-forming reactions. It also stereochemically catalyzes the radical polymerization of acrylates. Scandium(III) triflate complex of (4′S,5′S)-2,6-bis[4′-(triisopropylsilyl)oxymethyl-5′-phenyl-1′,3′-oxazolin-2′-yl]pyridine has been employed as catalyst for the asymmetric Friedel-Crafts reaction between substituted indoles and methyl (E)-2-oxo-4-aryl-3-butenoates.
Properties of Scandium trifluoromethanesulfonate
| Melting point: | >300 °C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Water (Slightly) |
| form | Powder |
| color | White |
| Water Solubility | Soluble in water, alcohol and acetonitrile. |
| Sensitive | Hygroscopic |
| Hydrolytic Sensitivity | 6: forms irreversible hydrate |
| BRN | 8510151 |
| Stability: | hygroscopic |
| InChI | InChI=1S/3CHF3O3S.Sc/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3 |
| CAS DataBase Reference | 144026-79-9(CAS DataBase Reference) |
Safety information for Scandium trifluoromethanesulfonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Scandium trifluoromethanesulfonate
| InChIKey | HZXJVDYQRYYYOR-UHFFFAOYSA-K |
| SMILES | C(F)(S([O-])(=O)=O)(F)F.C(F)(F)(F)S([O-])(=O)=O.C(F)(F)(F)S([O-])(=O)=O.[Sc+3] |
Scandium trifluoromethanesulfonate manufacturer
OctaneX Labs
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 144026-79-9 SCANDIUM TRIFLATE 99%View Details
144026-79-9 SCANDIUM TRIFLATE 99%View Details
144026-79-9 -
 144026-79-9 99%View Details
144026-79-9 99%View Details
144026-79-9 -
 Scandium(III) trifluoromethanesulfonate CAS 144026-79-9View Details
Scandium(III) trifluoromethanesulfonate CAS 144026-79-9View Details
144026-79-9 -
 144026-79-9 99%View Details
144026-79-9 99%View Details
144026-79-9 -
 Scandium(III) Trifluoromethanesulfonate CAS 144026-79-9View Details
Scandium(III) Trifluoromethanesulfonate CAS 144026-79-9View Details
144026-79-9 -
 Scandium(III) triflate, 99% CAS 144026-79-9View Details
Scandium(III) triflate, 99% CAS 144026-79-9View Details
144026-79-9 -
 Scandium(III) triflate 97% CAS 144026-79-9View Details
Scandium(III) triflate 97% CAS 144026-79-9View Details
144026-79-9 -
 Scandium(III) triflate CAS 144026-79-9View Details
Scandium(III) triflate CAS 144026-79-9View Details
144026-79-9
