Trimethylsilyl trifluoromethanesulfonate
Synonym(s):TMSOTf;Trimethylsilyl trifluoromethanesulfonate;TMS triflate;Trifluoromethanesulfonic acid trimethylsilylester;Trimethylsilyl triflate, Trifluoromethanesulfonic acid trimethylsilyl ester
- CAS NO.:27607-77-8
- Empirical Formula: C4H9F3O3SSi
- Molecular Weight: 222.26
- MDL number: MFCD00000406
- EINECS: 248-565-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
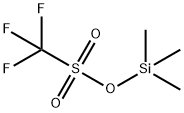
What is Trimethylsilyl trifluoromethanesulfonate?
Chemical properties
clear colourless to light brown fuming liquid
Physical properties
bp 45–47 °C/17 mmHg, 39–40 °C/12 mmHg; d 1.225 g cm?3.
The Uses of Trimethylsilyl trifluoromethanesulfonate
Trimethylsilyl Trifluoromethanesulfonate is a trialkylsilyl triflate used as a catalyst in organic synthesis. Trimethylsilyl Trifluoromethanesulfonate is used in combination with boron trifluoride eth yl ether to prepare a Lewis acid that is more powerful than its components and especially effective in acetonitrile solvent. Trimethylsilyl is a common reagent used in a Dieckmann-like cyclization of ester-imides and diesters.
The Uses of Trimethylsilyl trifluoromethanesulfonate
Catalyst and silylating agent for organic syntheses.
The Uses of Trimethylsilyl trifluoromethanesulfonate
Trimethylsilyl Trifluoromethane sulfonate is generally used following reactions:
1. Silylation. TMSOTf is widely used in the conversion of car bonyl compounds to their enol ethers. The conversion is some 109 faster with TMSOTf/triethylamine than with chlorotrimethy lsilane.Dicarbonyl compounds are converted to the corresponding bis enol ethers; this method is an improvement over the previous two step method.In general, TMSOTf has a tendency toC-silylation which is seen most clearly in the reaction of esters, whereC-silylation dominates over O-silylation.
2.Carbonyl Activation. 1,3-Dioxolanation of conjugated enals is facilitated by TMSOTf in the presence of 1,2-bis(trimethylsilyl oxy)ethane. In particular, highly selective protection of sterically differentiated ketones is possible (eq 10).TMSOTf mediates a stereoselective aldol-type condensation of silyl enol ethers and acetals (or orthoesters). The nonbasic reaction conditions are extremely mild. The use of TMSOTf in aldol reactions of silyl enol ethers and ketene acetals with aldehydes is ubiquitous. Stereoselective cyclization of α,β-unsaturated enamide esters is induced by TMSOTf and has been used as a route to quinolizidines and indolizidines.
3.The often difficult conjugate addition of alkynyl organometallic reagents to enones is greatly facilitated by TMSOTf. In particular, alkynyl zinc reagents (normally unreactive with α,β-unsaturated carbonyl compounds) add in good yield.The formation of nitrones by reaction of aldehydes and ketones with N-methyl-N,O-bis(trimethylsilyl)hydroxylamine is accelerated when TMSOTf is used as a catalyst; the acceleration is particularly pronounced when the carbonyl group is under a strong electronic influence.5. Methyl glucopyranosides and glycopyranosyl chlorides undergo allylation with allylsilanes under TMSOTf catalysis to give predominantly α-allylated carbohydrate analogs.Glycosidation is a reaction of massive importance and wide spread employment. TMSOTf activates many selective glycosidation reactions.
4.O-Silylation. The formation of TMS ethers can be achieved by reacting the requisite alcohol with TMSOTf and an amine (triethy lamine, pyridine, or 2,6-lutidine) in dichloromethane;C-Silylation. Depending on the reaction conditions, sec ondary amides can be either C-silylated or N-silylated;N-Silylation. The N-bis-silylation of α-amino acids with TMSOTf is only effective for glycine; for other α-amino acids N-mono-silylation prevails because the larger size of the carbon chain at the α-position hinders bis-silylation;C,O-Bis-silylation. Bis-silylation ofα,β-unsaturated carbonyl compounds can be achieved by palladium-TMSOTf-catalyzed addition of disilanes to enones, enals, or aromatic aldehydes via an η3-silyloxyallylpalladium intermediate;Carbonyl Activation. TMSOTf frequently acts as a Lewis acid and it is able to activate several functional groups (the car bonyl group, the acetal unit, the nitrone moiety,…) thus facilitating different kinds of reactions;Acetal Activation. TMSOTf acts as a catalyst for the addi tion of several nucleophiles (allylsilanes, allylstannanes, silyl enol ethers, trimethylsilyl cyanide) towardN,O-acetals;Nitrone Activation. The nucleophilic addition to aldonitrones depends on the nature of the metal involved and the presence/absence of an activator;Epoxide Ring Opening. One-pot alkylation-O-silylation re actions of epoxides take place in excellent yields;Cleavage of Protecting Groups. THP ethers of primary, sec ondary, and phenolic alcohols can be conveniently deprotected at room temperature; Hypervalent Iodine Chemistry. The formation of hyperva lent iodine complexes is often promoted by TMSOTf.
What are the applications of Application
Trimethylsilyl trifluoromethanesulfonate is a strong silylating agent
Synthesis
Trimethylsilyl trifluoromethanesulfonate is a frequent catalyzer or cationic initiator as a silylation reagent in organic synthesis; currently, the conventional preparation method has two kinds.
A kind of reaction is to prepare with tetramethylsilane by trifluoromethanesulfonic acid. However, tetramethylsilane in the preparation process often with a large amount of dimethylbutanes, dimethylchlorosilane, impurities such as dimethyl dichlorosilane (DMCS), above-mentioned impurity and trifluoromethanesulfonic acid is easy to occur side reaction, especially the trifluoromethanesulfonic acid dimethyl silicone grease that dimethylchlorosilane and trifluoromethanesulfonic acid generate and the boiling point of trimethylsilyl triflate are very approaching, be difficult to separate by the method for rectifying.
Another kind of preparation method is fluoroform sulphonate and silylation reagent reaction. This preparation method can obtain a purity of up to 99.9% Trimethylsilyl trifluoromethanesulfonate.
Properties of Trimethylsilyl trifluoromethanesulfonate
| Melting point: | 25°C |
| Boiling point: | 77 °C/80 mmHg (lit.) |
| Density | 1.228 g/mL at 25 °C (lit.) |
| vapor pressure | 4.7 hPa (20 °C) |
| refractive index | n |
| Flash point: | 78 °F |
| storage temp. | Store below +30°C. |
| solubility | sol aliphatic and aromatic hydrocarbons, haloalkanes,
ethers. |
| form | Fuming Liquid |
| color | Clear colorless to light brown |
| Specific Gravity | 1.15 |
| Water Solubility | REACTS |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Merck | 14,9719 |
| BRN | 1868911 |
| CAS DataBase Reference | 27607-77-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Trimethylsilyl trifluoromethanesulfonate(27607-77-8) |
| EPA Substance Registry System | Methanesulfonic acid, trifluoro-, trimethylsilyl ester (27607-77-8) |
Safety information for Trimethylsilyl trifluoromethanesulfonate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H226:Flammable liquids H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Trimethylsilyl trifluoromethanesulfonate
| InChIKey | FTVLMFQEYACZNP-UHFFFAOYSA-N |
Trimethylsilyl trifluoromethanesulfonate manufacturer
SAKEM LLP
Chimerical
GLR Innovations
Anurash Technologies Inc
SRF LIMITED
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 27607-77-8 98%View Details
27607-77-8 98%View Details
27607-77-8 -
 Trifluoromethanesulfonic acid trimethylsilyl ester 98%View Details
Trifluoromethanesulfonic acid trimethylsilyl ester 98%View Details -
 Trimethyl silyl triflate CAS 27607-77-8View Details
Trimethyl silyl triflate CAS 27607-77-8View Details
27607-77-8 -
 Trimethylsilyl Trifluoromethanesulfonate CAS 27607-77-8View Details
Trimethylsilyl Trifluoromethanesulfonate CAS 27607-77-8View Details
27607-77-8 -
 Trimethylsilyl trifluoromethanesulfonate CAS 27607-77-8View Details
Trimethylsilyl trifluoromethanesulfonate CAS 27607-77-8View Details
27607-77-8 -
 Trimethylsilyl trifluoromethanesulfonate CAS 27607-77-8View Details
Trimethylsilyl trifluoromethanesulfonate CAS 27607-77-8View Details
27607-77-8 -
 Trimethylsilyl Trifluoromethanesulfonate,Cas No-27607-77-8, GLR Innovations, Gm,KgsView Details
Trimethylsilyl Trifluoromethanesulfonate,Cas No-27607-77-8, GLR Innovations, Gm,KgsView Details
27607-77-8 -
 230 L 50 kg Trimethylsilyl Trifluoromethanesulfonate, 99%View Details
230 L 50 kg Trimethylsilyl Trifluoromethanesulfonate, 99%View Details
27607-77-8
