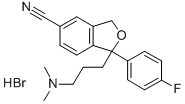
Escitalopram oxalate
Synonym(s):Escitalopram oxalate;S-(+)-1-[3-(Dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile oxalate;S-(+)-Citalopram oxalate
- CAS NO.:219861-08-2
- Empirical Formula: C22H23FN2O5
- Molecular Weight: 414.43
- MDL number: MFCD06407826
- EINECS: 620-544-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
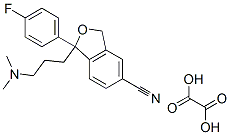
What is Escitalopram oxalate?
Description
Escitalopram was launched as Cipralex? in Switzerland, Sweden and UK for the treatment of depression and panic disorder. It is the S-enantiomer version of the selective serotonin reuptake inhibitor (SSRI) citalopram approved in 1989. It can be obtained from 5cyanophthalide by successive reactions with 4-fluorophenyl magnesium bromide and 3-(dimethylamino)propyl magnesium chloride. The resulting racemic diol can be resolved by several routes such as crystallization with a chiral acid. Finally, a two step cyclisation procedure affords escitalopram. Escitalopram is twice as effective as the racemate and over 100 fold more potent than the R-enantiomer in inhibiting the 5HT reuptake in vivo in rat brain synaptosomes. Moreover, it exhibits higher selectivity for the human serotonin transporter relative to the noradrenaline or dopamine transporters than any other currently available SSRl’s. In the mouse forced swim test, the duration of immobility (which reflects antidepressant activity) for escitalopram was comparable to citalopram and greater than (R)-citalopram. Clinical trials in patients with panic disorders or depression have shown that escitalopram has a clinically relevant and significant effect. Additionally, it has a faster onset of antidepressant action than citalopram. Escitalopram has linear pharmacokinetics, with a long half-life (27-32 h). It is extensively metabolized in the liver via cytochromes P450 to S(+)-desmethyl and S(+)-didesmethyl citalopram. However, it has been shown to be a weak or negligible inhibitor of CYP450 drugmetabolizing enzymes in vitro. Escitalopram is well tolerated with nausea being the most common side effect.
Chemical properties
White Solid
Originator
Lundbeck (Denmark)
The Uses of Escitalopram oxalate
Escitalopram oxalate may be used as a pharmaceutical primary standard for the quantification of the analyte in pharmaceutical formulations using analytical techniques.
The Uses of Escitalopram oxalate
A labelled inhibitor of serotonin (5-HT) uptake. Antidepressant
What are the applications of Application
(S)-Citalopram Oxalate is a potential serotonin (5-HT) uptake inhibitor
What are the applications of Application
(S)-Citalopram-d6 Oxalate is a useful deuterium-labeled derivative of Citalopram
brand name
Lexapro (Forest).
General Description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Escitalopram Oxalate, a highly selective serotonin re-uptake inhibitor antidepressant, is a pure S-enantiomer of the racemic, bicyclic pthalates derivatives of citalopram. It is mainly developed for the treatment of depression and anxiety disorders.
Biochem/physiol Actions
Escitalopram is a selective serotonin reuptake inhibitor (SSRI), the S-enantiomer and eutomer of citalopram.
Mechanism of action
Escitalopram is the S-enantiomer of citalopram and is the most selective of SSRIs. SSRIs' mechanism of action is exerted by binding to the sodium-dependant serotonin transporter protein (SERT) located in the presynaptic neuron. SERT works by re-uptaking serotonin from the synaptic cleft to the presynaptic neuron. When SERT is inactivated by escitalopram, this increases the amount of serotonin in the synaptic cleft.
Side Effects
The main side effect of escitalopram include: Nausea, dry mouth, trouble sleeping, constipation, tiredness, drowsiness, dizziness, and increased sweating may occur.
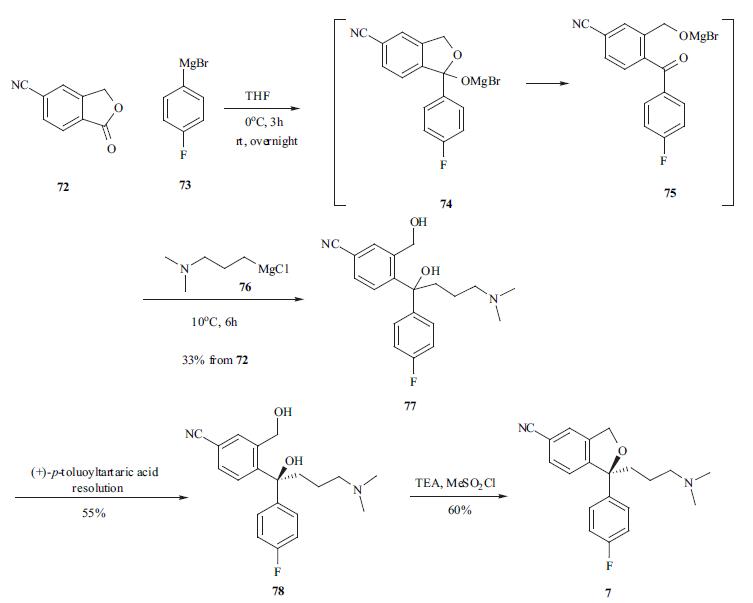
Synthesis
The synthesis of escitalopram was carried out in several different routes [30-33]. 5-Cyanophthalide (72) was treated with Grignard reagent 73 at 0??C to provide intermediate 75 which was reacted in situ with another Grignard reagent 76 to afford the diol in a one-pot process. Racemic diol 77 was resolved using (+)-p-toluoyltartaric acid to afford desired S isomer 78 in 55% yield. The ring closure reaction was carried out at 0??C using methanesulfonyl chloride in toluene to furnish escitalopram (7) in 60% yield.
storage
+4°C
References
[1] sánchez c1, bergqvist pb, brennum lt, gupta s, hogg s, larsen a, wiborg o. escitalopram, the s-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. psychopharmacology (berl). 2003 jun;167(4):353-62. epub 2003 apr 26.
Properties of Escitalopram oxalate
| Melting point: | 152-153°C |
| alpha | D +12.31° (c = 1 in methanol) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥15mg/mL |
| form | powder |
| color | white to tan |
| Merck | 14,2318 |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C20H21FN2O.C2H2O4/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20;3-1(4)2(5)6/h4-9,12H,3,10-11,14H2,1-2H3;(H,3,4)(H,5,6) |
| CAS DataBase Reference | 219861-08-2(CAS DataBase Reference) |
Safety information for Escitalopram oxalate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Escitalopram oxalate
| InChIKey | KTGRHKOEFSJQNS-UHFFFAOYSA-N |
| SMILES | C1(CCCN(C)C)(C2=CC=C(F)C=C2)C2=C(C=C(C#N)C=C2)CO1.C(O)(=O)C(O)=O |
Escitalopram oxalate manufacturer
SETV ASRV LLP
Cocreate Global Technologies Pvt Ltd
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
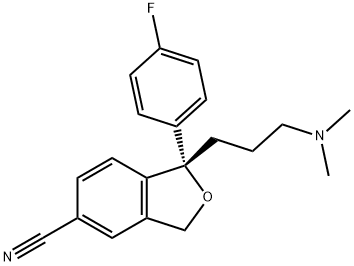

You may like
-
 219861-08-2 98%View Details
219861-08-2 98%View Details
219861-08-2 -
 Escitalopram oxalate 98%View Details
Escitalopram oxalate 98%View Details -
 ESCITALOPRAM OXALATE 219861-08-2 95-99%View Details
ESCITALOPRAM OXALATE 219861-08-2 95-99%View Details
219861-08-2 -
 Escitalopram oxalate 99%View Details
Escitalopram oxalate 99%View Details
219861-08-2 -
 Escitalopram oxalate 98%View Details
Escitalopram oxalate 98%View Details -
 Escitalopram oxalate 98%View Details
Escitalopram oxalate 98%View Details -
 Escitalopram oxalate 98.00% CAS 219861-08-2View Details
Escitalopram oxalate 98.00% CAS 219861-08-2View Details
219861-08-2 -
 (S)-Citalopram Oxalate CAS 219861-08-2View Details
(S)-Citalopram Oxalate CAS 219861-08-2View Details
219861-08-2
