Calcium oxalate
Synonym(s):Calcium oxalate (1:1)
- CAS NO.:563-72-4
- Empirical Formula: C2H4CaO4
- Molecular Weight: 132.13
- MDL number: MFCD00012474
- EINECS: 209-260-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-16 09:55:30
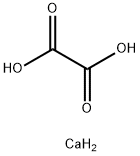
What is Calcium oxalate?
Description
Calcium oxalate (in archaic terminology, oxalate of lime) is a chemical compound that forms envelope-shaped crystals, known in plants as raphides. A major constituent of human kidney stones, the chemical is also found in beerstone, a scale that forms on containers used in breweries. Its chemical formula is CaC2O4 or Ca (COO)2.
Chemical properties
Calcium oxalate is white precipitate, insoluble in weak acids, but soluble in strong acids, formed by reaction of soluble calcium salt solution and ammonium oxalate solution. Solubility at 18 °C 0.0056 g anhydrous salt per liter of saturated solution.
Physical properties
Most crystals look like a 6 sided prism and often look like a pointed picket from a wooden fence. More than 90 % of the crystals in a urine sediment will have this type of morphology. These other shapes are less common than the 6 sided prism, however it is important to be able to quickly identify them in case of emergency.
What are the applications of Application
Calcium oxalate is used in the manufacture of ceramic glazes.
General Description
Purity based on trace metal analysis
Nature sources
Oalcium oxalate crystals are commonly associated with free-living, pathogenic, and plant-symbiotic fungi and are formed by the reprecipitation of solubilized calcium as the oxalate. Fungal-derived calcium oxalate can exhibit a variety of crystalline forms (tetragonal, bipyramidal, plate-like, rhombohedral, or needles), which often associate with the outer surfaces of hyphae.
Ecological function
The formation of calcium oxalate by fungi has an important influence on biological and geochemical processes in soils, acting as a reservoir for calcium in the ecosystem, and also influencing phosphate availability.
Properties of Calcium oxalate
| Melting point: | decomposes [CRC10] |
| Density | 2.2 g/mL at 25 °C (lit.) |
| form | Powder |
| color | White |
| Water Solubility | g/L solution H2O: 0.0069 (25°C), 0.0142 (95°C); solid phase, CaC2O4 ·H2O [KRU93]; soluble dilute HCl, HNO3 [HAW93] |
| Merck | 13,1690 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 563-72-4(CAS DataBase Reference) |
| EPA Substance Registry System | Ethanedioic acid, calcium salt (1:1) (563-72-4) |
Safety information for Calcium oxalate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Calcium oxalate
Calcium oxalate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 CALCIUM OXALATE 99%View Details
CALCIUM OXALATE 99%View Details -
 563-72-4 98%View Details
563-72-4 98%View Details
563-72-4 -
 Calcium oxalate 98%View Details
Calcium oxalate 98%View Details
563-72-4 -
 Calcium oxalate CAS 563-72-4View Details
Calcium oxalate CAS 563-72-4View Details
563-72-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
