Shikimic acid
Synonym(s):(-)-Shikimic acid;(3R,4S,5R)-(−)-3,4,5-Trihydroxy-1-cyclohexenecarboxylic acid;(3R,4S,5R)-3,4,5-Trihydroxy-1-cyclohexenecarboxylic acid
- CAS NO.:138-59-0
- Empirical Formula: C7H10O5
- Molecular Weight: 174.15
- MDL number: MFCD00066278
- EINECS: 205-334-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
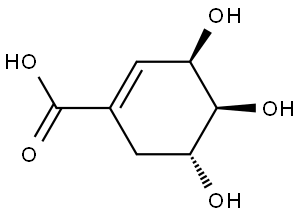
What is Shikimic acid?
Description
Shikimic acid (3,4,5-trihydroxy-1-cyclohexene-1-carboxylic acid) is a natural organic compound. Plant and microbial sources are the only sources of this compound. The isolation of shikimic acid from the fruit of Illicium religiosum was primarily reported by Ekmann in 1885. The name shikimic acid was derived from the oriental plant shikimi-no-ki in Japanese. It is now available in more quantities through the extraction from the fruit Illicium verum (Chinese star anise). This compound is generally utilized as a starting material for the industrial synthesis of the antiviral oseltamivir, a drug against the H1N1 influenza virus. It is also an essential intermediate in the biosynthesis of lignin, aromatic amino acids (phenylalanine, tyrosine, and tryptophan), and most alkaloids of plants and microorganisms[1]. The neuraminidase inhibitors oseltamivir or Tamiflu derived from the shikimic acid pathway are potent influenza viral neuraminidase inhibitors against most influenza strains ( influenza A and B infections). The mode of application is by mouth, either in the form of a pill or liquid.
Chemical properties
White Solid
The Uses of Shikimic acid
Shikimic acid has been used as a standard for the quantification of shikimate in apical parts of roots. It has also been used as a substrate in shikimate kinase assay.
The Uses of Shikimic acid
Naturally occurring (-)-form is a major biosynthetic precursor of phenylalanine, tyrosine, and tryptophan and hence of the majority of plant alkaloids. It is also involved in the biosynthesis of lignin, flavonpids and other important aromatic compounds.
What are the applications of Application
Shikimic acid is an intermediate in the biosynthesis of aromatic amino acids
Definition
ChEBI: A cyclohexenecarboxylic acid that is cyclohex-1-ene-1-carboxylic acid substituted by hydroxy groups at positions 3, 4 and 5 (the 3R,4S,5R stereoisomer). It is an intermediate metabolite in plants and micro rganisms.
Biochem/physiol Actions
Shikimic acid is observed to be carcinogenic in rat models, when administered. It serves as a precursor for the biosynthesis of aromatic amino acids, alkaloids and other aromatic metabolites in microorganisms. Shikimic acid is known to inhibit adenosine diphosphate (ADP) induced platelet aggregation and blood coagulation in rabbits.
References
[1] Priyanka Singh. “Shikimic acid as intermediary model for the production of drugs effective against influenza virus.” Phytochemicals as Lead Compounds for New Drug Discovery 14 1 (2020): 245–256.
Properties of Shikimic acid
| Melting point: | 185-187 °C (lit.) |
| Boiling point: | 225.11°C (rough estimate) |
| alpha | -180 º (c=4, H2O 25 ºC) |
| Density | 1.52 g/cm3 (27.2℃) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | -180 ° (C=1, H2O) |
| storage temp. | 2-8°C |
| solubility | 180g/l |
| form | Powder |
| pka | pK (14.1°) 5.19 |
| color | White to light beige or light gray |
| optical activity | [α]/D -180.0±5.0°, c = 4 in H2O |
| Water Solubility | 18 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8480 |
| BRN | 4782717 |
| CAS DataBase Reference | 138-59-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Shikimic acid(138-59-0) |
| IARC | 3 (Vol. 40, Sup 7) 1987 |
Safety information for Shikimic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Shikimic acid
| InChIKey | JXOHGGNKMLTUBP-HSUXUTPPSA-N |
Shikimic acid manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 (-)-Shikimic acid CAS 138-59-0View Details
(-)-Shikimic acid CAS 138-59-0View Details
138-59-0 -
 Shikimic acid 97% CAS 138-59-0View Details
Shikimic acid 97% CAS 138-59-0View Details
138-59-0 -
 Shikimic acid CAS 138-59-0View Details
Shikimic acid CAS 138-59-0View Details
138-59-0 -
 SHIKIMIC ACID 98%, Powder, 1 kgView Details
SHIKIMIC ACID 98%, Powder, 1 kgView Details
138-59-0 -
 Shikimic AcidView Details
Shikimic AcidView Details
138-59-0 -
 Shikimic Acid CAS 138-59-0View Details
Shikimic Acid CAS 138-59-0View Details
138-59-0 -
 Shikimic AcidView Details
Shikimic AcidView Details
138-59-0 -
 SHIKIMIC ACID, 98%View Details
SHIKIMIC ACID, 98%View Details
138-59-0
