Latanoprost
Synonym(s):Isopropyl (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]hept-5-enoate;Isopropyl (5Z,9α,11α,15R)-9,11,15-trihydroxy-17-phenyl-18,19,20-trinor-prost-5-en-1-oate;Latanoprost;Xalatan
- CAS NO.:130209-82-4
- Empirical Formula: C26H40O5
- Molecular Weight: 432.59
- MDL number: MFCD00216074
- EINECS: 634-172-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
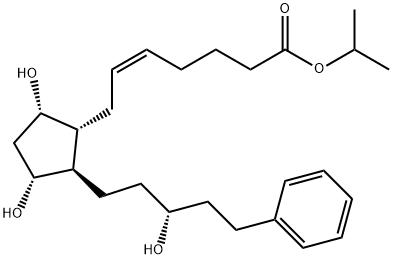
What is Latanoprost?
Absorption
This drug is rapidly absorbed in the cornea as an isopropyl ester prodrug and is then activated by the process of hydrolysis. A small amount of this drug is systemically absorbed. The Cmax of latanoprost in the systemic circulation is reached after 5 minutes and is measured to be 53 pg/mL. The Cmax in the aqueous humor is attained within 2 hours after administration. and has been estimated to be 15-30 ng/mL.
Toxicity
The oral LD50 in the rat is > 50 mg/kg.
An overdose of latanoprost is not expected to result in dangerous patient outcomes, however, conjunctival or episcleral hyperemia may occur. An intravenous infusion of 3 μg/kg of latanoprost in healthy volunteers led to mean plasma concentrations 200 times higher than a normally administered therapeutic dose and no adverse effects were noted. One study suggested that an overdose of latanoprost leads to cystoid macular edema after a large, unintended overdose. This resolved within 4 weeks after 4 weeks following treatment with nepafenac 0.3% eye drops in addition to oral acetazolamide. Contact the local poison control center for updated guidance on managing a latanoprost overdose.
Description
Xalatan was launched in Sweden, Switzerland and the US for the treatment of glaucoma. It can be synthesized, from the Corey lactone, using standard prostaglandin chemistry. Latanoprost is a PGF2α, analog that is more lipophilic thus is better able to penetrate the cornea. The (15R)-diastereomer has only 10% of the activity compared to the (15S)-diastereomer. It is a selective FP receptor agonist with little or no effect on the other prostanoid receptors. The antiglaucoma effects are the result of reduced intraocular pressure arising by increasing uveoscleral outflow with little or no effect on trabeculo-canalicular aqueous outflow and no effect on retinal vasculation or permeability of the blood-aqueous barrier. The topical application is sufficient for a single daily dosage and is well tolerated with no detectible conjunctival hyperaemia.
Chemical properties
Light Yellow Thick Oil
Originator
Pharmacia & pjohn (UK)
The Uses of Latanoprost
Latanoprost is the isopropyl ester of 17-phenyl-13,14-dihydro prostaglandin F2α (17-phenyl-13,14-dihydro PGF2α). It is a prodrug form of the free acid, a potent agonist of the FP receptor in the eye. It is an antifungal polyene macrolide active in the cell membrane soluble 5% Acetic acid. Latanoprost reduces intraocular pressure in glaucoma patients and treats glaucoma and other degenerative diseases of the eye with few side effects. The EC50 value of latanoprost (tested as the free acid) for FP receptors is 3.6 nM.
Indications
Latanoprost is indicated for the reduction of elevated intraocular pressure in patients who have been diagnosed with open-angle glaucoma or ocular hypertension. It is available as monotherapy or in a combination product with netarsudil or timolol.
In Canada, latanoprost is also indicated to treat elevated intraocular pressure due to angle-closure glaucoma that has been treated with peripheral iridotomy or laser iridoplasty.
Background
Latanoprost is a prodrug analog of prostaglandin F2 alpha that is used to treat elevated intraocular pressure (IOP). It was initially approved by the FDA in 1998. Latanoprost is the first topical prostaglandin F2 alpha analog used for glaucoma treatment. It has been found to be well-tolerated and its use does not normally result in systemic adverse effects like other drugs used to treat elevated intraocular pressure, such as Timolol. Another benefit latanoprost is that it can be administered once a day.
What are the applications of Application
Latanoprost is a prodrug of 17-phenyl-13,14-dihydro prostaglandin F2α
Definition
ChEBI: A prostaglandin Falpha that is the isopropyl ester prodrug of latanoprost free acid. Used in the treatment of open-angle glaucoma and ocular hypertension.
Manufacturing Process
Pyridinium p-toluenesulfonate (16 mg) was added to a stirred solution of 7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(3R)-5-phenyl-3-[(tetrahydro-2H-pyran-2-yl)oxy]pentyl]cyclopentyl]-5Z-heptenoic acid, isopropyl ester (0.7 g) in ethanol (20 mL) at room temperature. The mixture was stirred at 50° C. over 3 hours, by which time the reaction was complete (TLC monitoring). The mixture was concentrated under reduced pressure. The residue was diluted with dichloromethane (40 mL). The solution was washed with water (10 mL) and brine (10 mL), dried over sodium sulfate and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel (hexane/ethyl acetate 1:1 v/v) to obtain Latanoprost.
brand name
Xalatan (Pharmacia & Upjohn).
Therapeutic Function
Antiglaucoma
General Description
Latanoprost (Xalatan) is available as a 0.005% sterileophthalmic solution in a 2.5-mL dispenser bottle.Latanoprost is also marketed as a combination ophthalmicproduct with the β-adrenergic blocking agent, timolol,which apparently enhances IOP-lowering by decreasing theproduction of aqueous humor. Cautions and side effects aresimilar to those for other ophthalmic prostanoids.
Biochem/physiol Actions
Latanoprost is a potent, selective prostaglandin F2α?analog receptor agonist. It is hydrolyzed by esterases into its biologically active form latanoprost acid in the cornea. Latanoprostplays a role in reducing the intraocular pressure (IOP) due to which it has therapeutic effects in treating glaucoma.
Pharmacokinetics
Latanoprost effectively decreases intraocular pressure by increasing uveoscleral outflow. A decrease in intraocular pressure has been measured within 3–4 hours post-administration, reaches a maximum reduction at 8–12 hours, and can be maintained for 24 hours.
Safety Profile
A poison by ingestion and intravenous route. Human systemic effects. When heated to decomposition it emits acrid smoke and irritating vapors.
Side Effects
Between 3 to 10% of patients taking latanoprost have experienced iris pigmentation after about 3-4 months of latanoprost use. Patients should be notified of this risk before initiating treatment. It may occur in patients with light-coloured irides (green-brown or blue/grey-brown) or dark-coloured (brown) irides but is less pronounced in the latter group. This drug may also cause other ocular effects, including infrequent conjunctival hyperemia, pigmentation of periocular tissues, eyelash changes, hypertrichosis, and ocular irritation.
Metabolism
After corneal uptake, this prodrug is hydrolyzed and activated by esterases to become a pharmacologically active drug. The small portion of this drug that is able to reach the circulation is found to be metabolized by the liver to the 1,2-dinor and 1,2,3,4-tetranor metabolites through fatty acid beta-oxidation.
Properties of Latanoprost
| Boiling point: | 583.8±50.0 °C(Predicted) |
| alpha | D20 +31.57° (c = 0.91 in acetonitrile) |
| Density | 1.093±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: freely soluble |
| form | oil |
| pka | 14.84±0.70(Predicted) |
| color | pale yellow |
| Merck | 14,5376 |
| CAS DataBase Reference | 130209-82-4(CAS DataBase Reference) |
Safety information for Latanoprost
| Signal word | Warning |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Latanoprost
| InChIKey | GGXICVAJURFBLW-RDSJPUOVSA-N |
Latanoprost manufacturer
Century Pharmaceuticals Limited
Kavya Pharma
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

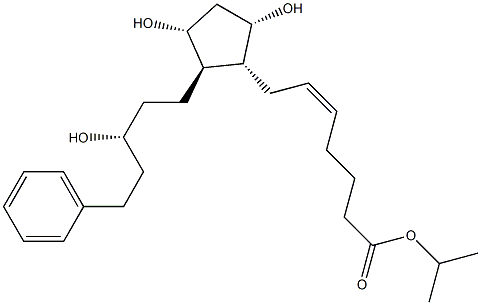
You may like
-
 Latanoprost. 130209-82-4 98%View Details
Latanoprost. 130209-82-4 98%View Details
130209-82-4 -
 130209-82-4 98%View Details
130209-82-4 98%View Details
130209-82-4 -
 Latanoprost CAS 130209-82-4View Details
Latanoprost CAS 130209-82-4View Details
130209-82-4 -
 Latanoprost CAS 130209-82-4View Details
Latanoprost CAS 130209-82-4View Details
130209-82-4 -
 Latanoprost USP CAS No 130209-82-4View Details
Latanoprost USP CAS No 130209-82-4View Details
130209-82-4 -
 Latanoprost Active pharmacetical IngredientView Details
Latanoprost Active pharmacetical IngredientView Details
130209-82-4 -
 Latanoprost Api, Grade Standard: Technical Grade, 130209-82-4View Details
Latanoprost Api, Grade Standard: Technical Grade, 130209-82-4View Details
130209-82-4 -
 Latanoprost Active Pharmaceutical Ingredients (API)View Details
Latanoprost Active Pharmaceutical Ingredients (API)View Details
130209-82-4
