Disulfur dichloride
Synonym(s):Disulfur dichloride;Sulfur chloride
- CAS NO.:10025-67-9
- Empirical Formula: Cl2S2
- Molecular Weight: 135.04
- MDL number: MFCD00011446
- EINECS: 233-036-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
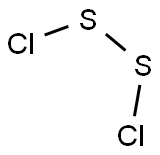
What is Disulfur dichloride?
Chemical properties
Disulfur dichloride is a fuming, oily liquid with a yellowish-red to amber color and a suffocating odor. Soluble in alcohol, ether, benzene, carbon disulfide, and amyl acetate; decomposes on contact withwater. Combustible. It has an added hazard since it oxidizes and hydrolyzes to sulfur dioxide and hydrogen chloride.
The Uses of Disulfur dichloride
Disulfur dichloride is used as Intermediate and chlorinating agent in the manufacture of organic chemicals, sulfur dyes, insecticides, synthetic rubbers; in cold vulcanization of rubber; as polymerization catalyst for vegetable oils; for hardening soft woods. The chemical fiber industry is used as a finishing agent for the manufacture of fabrics. The metallurgical industry is used as an extraction agent for precious and rare metals such as gold and silver.
Definition
Disulfur dichloride is a red fuming liquid with a strong smell. It is prepared by passing chlorine over molten sulfur and is used to harden rubber.
General Description
Sulfur monochloride appears as a yellow-red, oily, fuming liquid with a sharp odor. Contact or ingestion causes irritation or chemical burns to skin, eyes, and mucous membranes. Also poisonous by inhalation of vapors.
Health Hazard
Sulfur monochloride is an irritant of the eyes, mucous membranes, and skin. On contact with water, it decomposes to form hydrogen chloride and sulfur dioxide; because this occurs rapidly, it acts primarily as an upper respiratory irritant and does not ordinarily reach the lungs. However, exposure to high concentrations may cause pulmonary edema.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion and inhalation. A fuming, corrosive liquid very irritating to shin, eyes, and mucous membranes. It decomposes on contact with water to form the highly irritating hydrogen chloride, thiosulfuric acid, and sulfur. Its toxic effects are irritating to the upper respiratory tract, although the results of intoxication are usually transitory in nature. However, if hydrolysis is not complete in the upper respiratory tract, injury to the broncholes and alveoli can result. A fire hazard when in contact with organic matter, P203, Na2O2, water, Cr(OCl)2. Combustible when exposed to heat or flame. Will react with water or steam to produce heat and toxic and corrosive fumes. Can react with oxidizing materials. To fight fire, use CO2, dry chemical. When heated to decomposition it emits highly toxic fumes of Cland SOX.
Potential Exposure
Sulfur chloride finds use as a chlorinating agent, catalyst, and as an intermediate in the manufacture of organic chemicals; carbon tetrachloride; sulfur dyes; insecticides, synthetic rubber; and pharmaceuticals. Exposure may also occur during the extraction of gold, purification of sugar juice; finishing and dyeing textiles; processing vegetable oils; hardening wood; and vulcanization of rubber. Has been used as a military poison.
Shipping
UN1828 Sulfur chlorides, Hazard class: 8; Labels: 8-Corrosive material, Potential Inhalation Hazard (Special Provision 5)
Purification Methods
It isa pungent, irritating golden yellow liquid. When impure its colour is orange to red due to SCl2 formed. It fumes in moist air and liberates HCl, SO2 and H2S in the presence of H2O. Distil it and collect the fraction boiling above 137o at atmospheric pressure. Fractionate this fraction over sulfur at ca 12mm using a ground glass apparatus (b 29-30o). Alternatively purify it by distillation below 60o from a mixture containing sulfur (2%) and activated charcoal (1%), under reduced pressure (e.g. 50mm). It is soluble in EtOH, *C6H6, Et2O, CS2 and CCl4. Store it in a closed container in the dark in a refrigerator. [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 371 1963.] HARMFUL VAPOURS.
Incompatibilities
Decomposes violently in water, forming hydrochloric acid, sulfur dioxide; sulfur, sulfite, thiosulfate, and hydrogen sulfide. Reacts with oxidizers, strong bases; peroxides, phosphorus oxides; organics, antimony, antimony sulfide; arsenic sulfide; mercury oxide; tin, alkenes, terpenes, unsaturated glycerides; chromyl chloride; methyl sulfoxide; dimethylformamide, acetone, and other compounds; causing fire and explosion hazard. Corrosive to many metals in presence of water. Attacks some plastics, rubber and coatings.
Waste Disposal
Wearing protective equipment, spray carefully onto sodium ash/slaked lime mixture. Then spray with water, dilute, neutralize and flush to drain.
Precautions
Sulfur monochloride is minimally corrosive to carbon steel and iron when dry. When wet, it behaves like hydrochloric acid and attacks steel, cast iron, aluminum, stainless steels, copper and copper alloys, and many nickel-based materials.
Properties of Disulfur dichloride
| Melting point: | −80 °C(lit.) |
| Boiling point: | 138 °C(lit.) |
| Density | 1.688 g/mL at 25 °C(lit.) |
| vapor density | 4.7 (vs air) |
| vapor pressure | 6.8 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | >130℃ |
| storage temp. | Store below +30°C. |
| solubility | soluble in No data available |
| form | yellow-red oily liquid |
| color | yellow-red oily liquid |
| explosive limit | 4.2-32.5%(V) |
| Water Solubility | reacts |
| Merck | 13,9060 |
| Dielectric constant | 4.8(15℃) |
| CAS DataBase Reference | 10025-67-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Disulfur dichloride(10025-67-9) |
| EPA Substance Registry System | Sulfur monochloride (10025-67-9) |
Safety information for Disulfur dichloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Disulfur dichloride
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 10025-67-9 Sulfur monochloride 98%View Details
10025-67-9 Sulfur monochloride 98%View Details
10025-67-9 -
 Sulphur monochloride CAS 10025-67-9View Details
Sulphur monochloride CAS 10025-67-9View Details
10025-67-9 -
 SULPHUR MONOCHLORIDE For Synthesis CAS 10025-67-9View Details
SULPHUR MONOCHLORIDE For Synthesis CAS 10025-67-9View Details
10025-67-9 -
 Disulfur dichloride CAS 10025-67-9View Details
Disulfur dichloride CAS 10025-67-9View Details
10025-67-9 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
