10025-67-9
Product Name:
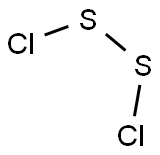
Disulfur dichloride
Formula:
Cl2S2
Synonyms:
Disulfur dichloride;Sulfur chloride
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sulfur monochloride appears as a yellow-red, oily, fuming liquid with a sharp odor. Contact or ingestion causes irritation or chemical burns to skin, eyes, and mucous membranes. Also poisonous by inhalation of vapors. |
|---|---|
| Color/Form | Light amber to yellowish red oily liquid |
| Odor | Suffocating odor |
| Boiling Point | 280 °F at 760 mmHg (USCG, 1999) |
| Melting Point | -112 °F (USCG, 1999) |
| Flash Point | 245 °F (USCG, 1999) |
| Solubility | Decomposes (NIOSH, 2023) |
| Density | 1.68 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | 4.66 (Air = 1) |
| Vapor Pressure | 7 mmHg (NIOSH, 2023) |
| Stability/Shelf Life | Stable at ambient temperature |
| Autoignition Temperature | 453 °F (USCG, 1999) |
| Decomposition | SULFUR CHLORIDE /WHEN HEATED IS/ ... ASSOCIATED WITH EVOLUTION OF DANGEROUS DECOMP PRODUCTS, SULFUR DIOXIDE & HYDROGEN CHLORIDE. |
| Viscosity | 0.978 cP |
| Corrosivity | Sulfur monochloride is minimally corrosive to carbon steel and iron when dry. When wet, it behaves like hydrochloric acid and attacks steel, cast iron, aluminum, stainless steels, copper and copper alloys, and many nickel-based materials. |
| Heat of Vaporization | 3.6401X10+4 J/mole |
| Surface Tension | 42.5 mN/m at 25 °C |
| Ionization Potential | 9.40 eV |
| Odor Threshold | Air: 0.77 ul/l; Odor safety class C; C= less than 50% of distracted persons perceive warning of TLV. /Hydrochloric acid/ |
| Refractive Index | Index of refraction: 1.670 at 20 °C/D |
| Other Experimental Properties | 1 mg/cu m= 0.2 ppm & 1 ppm= 5.5 mg/cu m @ 25 °C, 760 mm Hg |
| Chemical Classes | Toxic Gases & Vapors -> Other Toxic Gases & Vapors |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 135.0 g/mol |
|---|---|
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 133.8818477 g/mol |
| Monoisotopic Mass | 133.8818477 g/mol |
| Topological Polar Surface Area | 50.6 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sulfur monochloride appears as a yellow-red, oily, fuming liquid with a sharp odor. Contact or ingestion causes irritation or chemical burns to skin, eyes, and mucous membranes. Also poisonous by inhalation of vapors.
