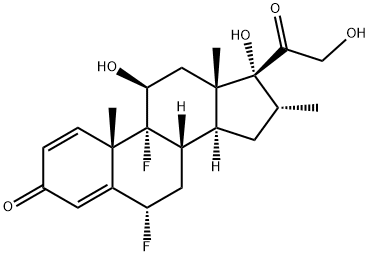
Diflucortolone
- CAS NO.:2607-06-9
- Empirical Formula: C22H28F2O4
- Molecular Weight: 394.46
- MDL number: MFCD00867459
- EINECS: 220-022-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:36
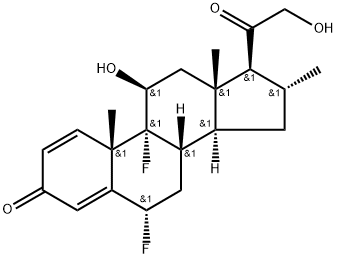
What is Diflucortolone?
Absorption
The absorption of diflucortolone is made mainly percutaneously but it may be absorbed systemically. The absorption and bioavailability of diflucortolone will be related to the type of formulation found in the medication. The percutaneous absorption depends on the vehicle, dose, treatment area, duration of treatment, the condition of treatment, the status of penetration barrier and localization of treated area in the body. Thus, rectal administration of diflucortolone produces a slow and low absorption with an AUC, Cmax and Tmax of 10.8 ng h/ml, 0.75 ng/ml and 4.7 h, respectively.
Toxicity
Diflucortolone can cause skin irritation, vesicles or red patches on the skin.
Originator
Nerisone,Schering,UK,1976
The Uses of Diflucortolone
Glucocorticoid. .
The Uses of Diflucortolone
Difluocortolone is used as a topical therapy in the treatment of pediatric tinea corporis; a common mycotic infection in children.
Indications
Difluocortolone is used as a topical treatment of the symptoms of inflammatory skin disorders like eczema, seborrheic eczema, lichen planus and psoriasis. All these disorders present as a common characteristic the occurrence of symptoms as itching, swelling, redness and scaling.
Background
Difluocortolone is a potent topical corticosteroid. It is commonly used in dermatology for the reduction of inflammation and itching. It was submitted to the FDA in July 1984 by the pharmaceutical company Schering AG.
Definition
ChEBI: Diflucortolone is a 21-hydroxy steroid.
Manufacturing Process
16α-Methyl-6α,9α-difluoro-δ4-pregnene-11α,21-diol-3,20-dione-21-acetate
(MP = 229°/232°-234°C (with decomposition) is dehydrogenated in 1.2-
position by means of Bacillus lentus, Mutant MB 284, whereby the 21-acetate
group is simultaneously saponified. (It is possible under the same conditions
to start with the free 21-hydroxyl compound.)
For this purpose a fermenter made of stainless steel having a 50 liter capacity
is charged with 30 liters of a nutrient solution of 0.1% yeast extract, 0.5%
cornsteep and 0.2% glucose, heated for one-half hour at 120°C for
sterilization purposes, and after cooling, inoculated with a bacterial suspension
of Bacillus lentus MB 284.
After 24 hours of growth at 28°C under stirring (220 revolutions per minute)
and aeration (1.65 m3/hr), 1.8 liters of the obtained culture is removed under
sterile conditions and transferred with 28 liters of the same sterilized nutrient
medium into a fermenter of the same size.
Simultaneously, 6 g of 16α-methyl-6α,9α-difluoro-δ4-pregnene-11β,21-diol3,20-dione-21-acetate in 200 cc of dimethylformamide are added and the
fermentation is continued for 50 hours under the same conditions.
The course of the fermentation is tested by removal of samples which are
extracted with methyl isobutyl ketone. The extracts are analyzed by thin layer
chromatography using a system of benzene/ethyl acetate (4:1).
After further working up there is obtained an oily crystalline residue which is
subjected to chromatography on silica gel. The 16α-methyl-6α,9α-difluoroδ1,4-pregnadien-11β,21-diol-3,20-dione is eluated with ethyl acetatechloroform (1:2), it is recrystallized from ethyl acetate/ether and then formed
to melt at 240°/242°-244°C. The yield is 60% of the theoretical. The product
is reacted with valeric acid chloride to give the valerate ester.
Therapeutic Function
Antiinflammatory
Pharmacokinetics
Diflucortolone is a steroid with the properties of being an anti-inflammatory, antipruritic and vasoconstrictive. Its activity causes the vasoconstriction of the blood vessels and thus a decrease in the release of inflammatory substances. These actions produce the effect of skin soothed and elimination of the symptoms.
Metabolism
The metabolism of diflucortolone is done in the liver where it is very rapidly degraded. After 5 minutes of administration of diflucortolone in a dose of 1mg, there is a concentration of intact diflucortolone in plasma of 6-8 ng/ml. The analysis of the metabolites showed the presence of 11-keto-diflucortolone as the major metabolite in the plasma.
Properties of Diflucortolone
| Melting point: | 240-244°; mp 248-249° |
| alpha | D22 +111° (methanol) (Kieslich, 1976) |
| Boiling point: | 534.0±50.0 °C(Predicted) |
| Density | 1.29±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Slightly, Sonicated), DMSO (Slightly) |
| form | Off-White to Pale Grey Soild |
| pka | 12.90±0.70(Predicted) |
| CAS DataBase Reference | 2607-06-9(CAS DataBase Reference) |
Safety information for Diflucortolone
Computed Descriptors for Diflucortolone
Diflucortolone manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran


You may like
-
 Diflucortolone 99%View Details
Diflucortolone 99%View Details -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0 -
 124371-59-1 98%View Details
124371-59-1 98%View Details
124371-59-1 -
 53857-52-2 98%View Details
53857-52-2 98%View Details
53857-52-2