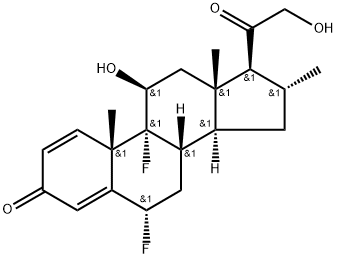
Diflucortolone valerate
- CAS NO.:59198-70-8
- Empirical Formula: C27H36F2O5
- Molecular Weight: 478.57
- MDL number: MFCD00468095
- EINECS: 261-655-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-01 18:08:31
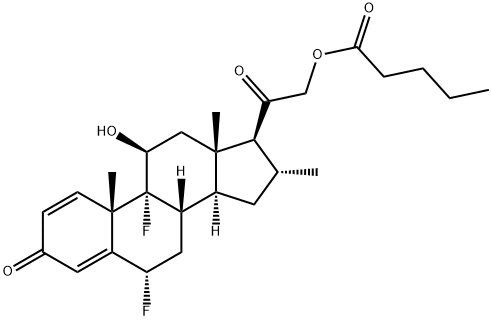
What is Diflucortolone valerate?
Originator
Nerisone,Schering,UK,1976
The Uses of Diflucortolone valerate
The 9α-fluoro derivative of Fluocortolone (F500500). Anti-inflammmatory.
Definition
ChEBI: Diflucortolone valerate is a corticosteroid hormone.
Manufacturing Process
16α-Methyl-6α,9α-difluoro-δ4-pregnene-11α,21-diol-3,20-dione-21-acetate
(MP = 229°/232°-234°C (with decomposition) is dehydrogenated in 1.2-
position by means of Bacillus lentus, Mutant MB 284, whereby the 21-acetate
group is simultaneously saponified. (It is possible under the same conditions
to start with the free 21-hydroxyl compound.)
For this purpose a fermenter made of stainless steel having a 50 liter capacity
is charged with 30 liters of a nutrient solution of 0.1% yeast extract, 0.5%
cornsteep and 0.2% glucose, heated for one-half hour at 120°C for
sterilization purposes, and after cooling, inoculated with a bacterial suspension
of Bacillus lentus MB 284.
After 24 hours of growth at 28°C under stirring (220 revolutions per minute)
and aeration (1.65 m3/hr), 1.8 liters of the obtained culture is removed under
sterile conditions and transferred with 28 liters of the same sterilized nutrient
medium into a fermenter of the same size.
Simultaneously, 6 g of 16α-methyl-6α,9α-difluoro-δ4-pregnene-11β,21-diol3,20-dione-21-acetate in 200 cc of dimethylformamide are added and the
fermentation is continued for 50 hours under the same conditions.
The course of the fermentation is tested by removal of samples which are
extracted with methyl isobutyl ketone. The extracts are analyzed by thin layer
chromatography using a system of benzene/ethyl acetate (4:1).
After further working up there is obtained an oily crystalline residue which is
subjected to chromatography on silica gel. The 16α-methyl-6α,9α-difluoroδ1,4-pregnadien-11β,21-diol-3,20-dione is eluated with ethyl acetatechloroform (1:2), it is recrystallized from ethyl acetate/ether and then formed
to melt at 240°/242°-244°C. The yield is 60% of the theoretical. The product
is reacted with valeric acid chloride to give the valerate ester.
Therapeutic Function
Antiinflammatory
Properties of Diflucortolone valerate
| Melting point: | 220°C |
| Boiling point: | 578.5±50.0 °C(Predicted) |
| alpha | D22 +100.8° (dioxane) |
| Density | 1.1227 (estimate) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Slightly), Dioxane (Slightly), Methanol (Slightly) |
| pka | 12.89±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
Safety information for Diflucortolone valerate
Computed Descriptors for Diflucortolone valerate
Diflucortolone valerate manufacturer
Stermone Chemicals Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![Ethyl (S)-9,10-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylate](https://img.chemicalbook.in/CAS/GIF/106939-34-8.gif)
You may like
-
 59198-70-8 Diflucortolone valerate 98%View Details
59198-70-8 Diflucortolone valerate 98%View Details
59198-70-8 -
 Diflucortolone valerate CAS 59198-70-8View Details
Diflucortolone valerate CAS 59198-70-8View Details
59198-70-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8