Fluorometholone
Synonym(s):11β,17α-Dihydroxy-9-fluoro-6-methyl-1,4-pregnadiene-3,20-dione
- CAS NO.:426-13-1
- Empirical Formula: C22H29FO4
- Molecular Weight: 376.46
- MDL number: MFCD00056461
- EINECS: 207-041-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
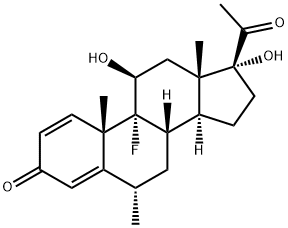
What is Fluorometholone?
Toxicity
Side effects may include acute anterior uveitis and perforation of the globe. Keratitis, conjunctivitis, corneal ulcers, mydriasis, conjunctival hyperemia, loss of accommodation and ptosis have occasionally been reported following local use of corticosteroids. LD50 = 234 mg/kg (rats)
Chemical properties
White powder
Originator
Oxylone, Upjohn, US,1959
The Uses of Fluorometholone
Fluorometholone is a glucocorticoid; anti-inflammatory.
Background
A glucocorticoid employed, usually as eye drops, in the treatment of allergic and inflammatory conditions of the eye. It has also been used topically in the treatment of various skin disorders. (From Martindale, The Extra Pharmacopoeia, 30th ed, p732)
Indications
For the ophthalmic treatment of corticosteroid-responsive inflammation of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe.
Definition
ChEBI: A member of the class of glucocorticoids that is Delta1-progesterone substituted at positions 11beta and 17 by hydroxy groups, at position 6alpha by a methyl group and at position 9 by a fluoro group. Used for the treatment of corticosteroid-responsive inflammation of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe.
Manufacturing Process
The following description is taken from US Patent 2,867,637.
(a) Preparation of 6α-Methyl-9α-Fluoro-11β,17α,21-Trihydroxy-1,4Pregnadiene-3,20-Dione 21-Methanesulfonate: A solution was prepared containing 250 mg of 1-dehydro-6α-methyl-9α-fluorohydrocortisone [G.B. Spero et al, J. Am. Chem. Soc.79, 1515 (1957)] in 6 ml of pyridine. This solution was cooled to 0°C and treated with 0.25 ml of methanesulfonyl chloride. Thereafter the solution was allowed to stir at a temperature between 0° and 5°C for a period of 18 hours. Thereafter ice and 2 ml of water were added, followed by 30 ml of sufficient dilute (5%) hydrochloric acid to neutralize the pyridine. The mixture was then filtered, the precipitate washed with water and dried to give 197 mg of crude 6α-methyl-9α-fluoro11β,17α,21-trihydroxy-1,4-pregnadiene-3,20-dione 21-methanesulfonate of MP 165° to 185°C.
(b) Preparation of 6α-Methyl-9α-Fluoro-11β,17α-Dihydroxy-21-Iodo-1,4Pregnadiene-3,20-Dione: The crude 197 mg of methanesulfonate of 6αmethyl-9α-fluoro-11β,17α,21-trihydroxy-1,4-pregnadiene-3,20-dione was dissolved in 5 ml of acetone and treated with a solution of 197 mg of sodium iodide in 5 ml of acetone. The mixture was heated under reflux with stirring for a period of 15 minutes. The heating was then discontinued and the mixture concentrated to dryness at reduced pressure to give 6α-methyl-9αfluoro-11β,17α-dihydroxy-21-iodo-1,4-pregnadiene-3,20-dione.
(c) Preparation of 6α-Methyl-9α-Fluoro-11β,17α-Dihydroxy-1,4-Pregnadiene3,20-Dione: The crude 6α-methyl-9α-fluoro-11β,17α-dihydroxy-21-iodo-1,4pregnadiene-3,20-dione was slurried with 5 ml of acetic acid and stirred for a period of 45 minutes. Thereafter was added a solution of 250 mg of sodium thiosulfate pentahydrate in 5 ml of water causing the iodine color to disappear. Additional water was added (30 ml) and the reaction mixture was filtered. The resulting solid precipitate was washed with water and dried to give 146 mg of crude 6α-methyl-9α-fluoro-11β,17α-dihydroxy-1,4pregnadiene-3,20-dione.
The crude material was then chromatographed by dissolving 120 mg of 6αmethyl-9α-fluoro-11β,17α-dihydroxy-1,4-pregnadiene-3,20-dione in 300 ml of methylene chloride and allowing the thus obtained solution to be absorbed by a chromatographic column containing 10 grams of Florisil anhydrous magnesium silicate. The column was developed taking fractions of 20 ml each as follows:Fractions 11 through 24 inclusive were combined, evaporated and twice recrystallized from acetone to give pure 6α-methyl-9α-fluoro-11β,17αdihydroxy-1,4-pregnadiene-3,20-dione of melting point 292° to 303°C.
brand name
Flarex (Alcon).
Therapeutic Function
Glucocorticoid, Antiinflammatory
General Description
Fluorometholone, 9-fluoro-11β,17-dihydroxy-6α-methylpregn-4-ene-3,20-dione(Fluor-Op, FML), lacks the typical C21 OH group of GCsand is used exclusively in ophthalmic products. The 17-acetateof fluorometholone is also used as an ophthalmic suspension(Flarex).
Biochem/physiol Actions
Clinically significant in allergic conjunctivitis and as anti-inflammatory following cataract surgery.
Pharmacokinetics
Corticosteroids such as fluorometholone inhibit the inflammatory response to a variety of inciting agents and probably delay or slow healing. They inhibit the edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation.
Metabolism
Not Available
Properties of Fluorometholone
| Melting point: | 292-303° |
| Boiling point: | 527.1±50.0 °C(Predicted) |
| alpha | +52~+60°(c=1, pyridine) (D/20℃) |
| Density | 1.0573 (rough estimate) |
| refractive index | 55 ° (C=1, Pyridine) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly), Pyridine (Slightly) |
| form | neat |
| pka | 12.50±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | 30mg/L(25 ºC) |
| Merck | 14,4175 |
| CAS DataBase Reference | 426-13-1(CAS DataBase Reference) |
| EPA Substance Registry System | Fluorometholone (426-13-1) |
Safety information for Fluorometholone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Fluorometholone
Fluorometholone manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 426-13-1 Fluorometholone 98%View Details
426-13-1 Fluorometholone 98%View Details
426-13-1 -
 426-13-1 98%View Details
426-13-1 98%View Details
426-13-1 -
 Fluorometholone 98%View Details
Fluorometholone 98%View Details
426-13-1 -
 Fluorometholone 426-13-1 99%View Details
Fluorometholone 426-13-1 99%View Details
426-13-1 -
 Fluorometholone 99%View Details
Fluorometholone 99%View Details -
 Fluorometholone CAS 426-13-1View Details
Fluorometholone CAS 426-13-1View Details
426-13-1 -
 Fluorometholone CAS 426-13-1View Details
Fluorometholone CAS 426-13-1View Details
426-13-1 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
