Dicamba
Synonym(s):3,6-Dichloro-o-anisic acid;3,6-Dichloro-2-methoxybenzoic acid
- CAS NO.:1918-00-9
- Empirical Formula: C8H6Cl2O3
- Molecular Weight: 221.04
- MDL number: MFCD00055283
- EINECS: 217-635-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
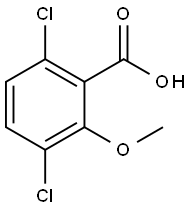
What is Dicamba?
Description
Dicamba was introduced in the 1960s and is selective in cereals, corn (Zea mays), sugar cane (Saccharum spp.), asparagus (Asparagus officinalis), and turf for the pre- and post-emergence control of annual and perennial broadleaf weeds. Dicamba exhibits lowto- moderate persistence in most soils. See Table 10 for the nomenclature, chemical structure, and physical and chemical properties of dicamba.
The Uses of Dicamba
Dicamba is mainly used as an herbicide to control weeds, dock, bracken, and brush. Dicamba is frequently applied with other herbicides, including atrazine, glyphosate, imazethapyr, ioxynil, and mecoprop.
The Uses of Dicamba
Herbicide.
The Uses of Dicamba
Selective, systemic preemergence and postemergence herbicide used to control both annual and perennial broad-leaved weeds, chickweed, mayweed and bindweed in cereals and other related crops.
What are the applications of Application
Dicamba is a herbicide derived from benzoic acid
Definition
ChEBI: A methoxybenzoic acid that is O-methylsalicylic acid substituted by chloro groups at positions 3 and 6.
General Description
Dicamba is a white solid dissolved in a liquid carrier. The carrier is water emulsifiable. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Since Dicamba is a liquid Dicamba can easily penetrate the soil and contaminate groundwater and nearby streams. Dicamba can cause illness by inhalation, skin absorption and/or ingestion. Dicamba is used as a herbicide.
Reactivity Profile
A halogenated benzoic acid derivative. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acids dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Dicamba to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Health Hazard
SOLID: Harmful if swallowed.
Fire Hazard
Not flammable.
Agricultural Uses
Herbicide: Used to control annual and perennial broadleaf weeds in corn, sorghum, small grains, pastures, hay, rangeland, sugarcane, asparagus, turf, grass-seed crops, and non-croplands. It can be applied to the leaves or to the soil. Dicamba controls annual and perennial broadleaf weeds in grain crops and grasslands, and it is used to control brush and bracken in pastures. It will kill broadleaf weeds before and after they sprout. Legumes will be killed by dicamba. In combination with a phenoxyalkanoic acid or other herbicide, dicamba is used in pastures, range land, and non-crop areas such as fence-rows and roadways to control weeds.
Trade name
BANEX®; BANLEN®; BANVEL®; BANVEL 4S®; BANVEL 4WS®; BANVEL CST®; BANVEL HERBICIDE®; BANVEL II HERBICIDE®; BRUSH BUSTER®; BUSHWHACKER®; CADENCE®; CASWELL No. 295®; CLARITY®; COMPOUND B DICAMBA®; DIANATE®; DISTINCT®; DYVEL®; FALLOWMASTER®; FLOWMASTER®; GORDON’S TRIGUARD®; GORDON’S TRI-MEC®; MARKSMAN®; MEDIBEN®; NORTHSTAR®; SUMMIT®; TARGET®; TRACKER®; TROOPER®; VANQUISH®; VELSICOL 58-CS-11®; VELSICOL COMPOUND R®; WEEDMASTER®; YUKON®
Biochem/physiol Actions
Dicamba is a broad leaf growth regulator that mimics plant growth auxins. Dicamba is used as a herbicide and is effective against glyphosate-resistant (GR) giant ragweed.
Pharmacology
Dicamba is highly mobile in soils and will leach or move upward depending on the flux of the soil water. Adsorption to soils is generally limited, although a few studies using acidic kaolinite and muck soils showed that dicamba was adsorbed to these soils. Adsorption of dicamba is greatest at low soil pHs and is minimal at pHs greater than 6.0. Because dicamba is highly water soluble, it is reasonable to expect that some loss may occur via soil water runoff from the application zone. However, studies conducted by Trichelle et al. (44) showed that such losses were minimal, i.e., less than 5.5% of applied. The rate of dicamba volatilization is not clear, although it is likely that it does occur to some extent. On planchets, approximately 50% of applied dicamba volatilized over a period of 11 weeks. The significance of this result is questionable, because in a similar study using soil, there was no appreciable volatilization (45).
Safety Profile
Moderately toxic by ingestion. Mutation data reported. When heated to decomposition it emits toxic fumes of Cl-.
Potential Exposure
AgriculturalChemical; Mutagen. Those involved in manufacture, formulation, and application of this postemergence herbicide. Used to control allual and perennial broad leafweeds in corn, sorghum, small grain pastures, andnoncroplands.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Environmental Fate
Biological. In a model ecosystem containing sand, water, plants and biota, dicamba
was slowly transformed to 5-hydroxydicamba (10% after 32 days) which slowly underwent
decarboxylation (Yu et al., 1975).
Soil. Smith (1974) studied the degradation of 14C-ring- and 14C-carboxyl-labeled
dicamba in moist prairie soils at 25°C. After 4 weeks, >50% of the herbicide degraded to
the principal products 3,6-dichlorosalicylic acid and carbon dioxide (Smith, 1974).
The half-lives for dicamba in soil incubated in the laboratory under aerobic conditions
ranged from 0 to 32 days (Altom and Stritzke, 1973; Smith, 1973, 1974; Smith and
Cullimore, 1975). In field soils, the half-lives for dicamba ranged from 6 to 10 days with
an average half-life of 7 days (Scifres and Allen, 1973; Stewart and Gaul, 1977). The
mineralization half-lives for dicamba in soil ranged from 147 to 309 days (Smith, 1974;Smith and Cullimore, 1975). In a Regina heavy clay, the loss of dicamba was rapid.
Approximately 10% of the applied dosage was recovered after 5 weeks. At the end of 5
weeks, approximately 28% was transformed to 3,6-dichlorosalicylic acid and carbon
dioxide (Smith, 1973a).
Groundwater. According to the U.S. EPA (1986) dicamba has a high potential to leach
to groundwater.
Plant. Dicamba is hydrolyzed in wheat and Kentucky bluegrass plants to 5-hydroxy-
2-methoxy-3,6-dichlorobenzoic acid and 3,6-dichlorosalicylic acid at yields of 90 and 5%,
respectively. The remaining 5% was unreacted dicamba (Broadhurst et al., 1966). Dicamba
was absorbed from treated soils, translocated in corn plants and then converted to 3,6-
dichlorosalicylic acid, p-aminobenzoic acid and benzoic acid (Krumzdorf, 1974).
Photolytic. When dicamba on silica gel plates was exposed to UV radiation (λ= 254
nm), it slowly degraded to the 5-hydroxy analog and water solubles (Humburg et al., 1989).
Chemical/Physical. Reacts with alkalies (Hartley and Kidd, 1987), amines and alkali
metals (Worthing and Hance, 1991) forming very water-soluble salts.
When dicamba was heated at 900°C, carbon monoxide, carbon dioxide, chlorine,
hydrochloric acid, oxygen and ammonia were produced (Kennedy et al., 1972, 1972a).
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with dicamba you should be trained on its proper handling and storage.Store in tightly closed containers in a cool, well-ventilatedarea away from incompatible materials listed above, heat,and water.
Shipping
Benzoic derivative pesticides, solid, toxic, n.o.s.require a label of “POISONOUS/TOXIC MATERIALS.” Itfalls in Hazard Class 6.1
Toxicity evaluation
There is very little metabolism of dicamba in mammals, and most is excreted unchanged in the urine. For example, rat excreted 96% of ingested 14C-dicamba after 24 hours (46). The acute oral LD50 for rat is 1707 mg/kg.
Mode of action
DICAMBA HD 5 is readily absorbed by plants through shoot and root uptake, translocates throughout the plant's system, and accumulates in areas of active growth. This product interferes with the plant's growth hormones (auxins) resulting in death of many broadleaf weeds.
Incompatibilities
Incompatible with sulfuric acid, bases,ammonia, aliphatic amines, alkanolamines, isocyanates,alkylene oxides, epichlorohydrin. Dicamba decomposes inheat, producing toxic and corrosive fumes including hydrogen chloride
Properties of Dicamba
| Melting point: | 112-116 °C (lit.) |
| Boiling point: | 316.96°C (rough estimate) |
| Density | 1.57 |
| refractive index | 1.5000 (estimate) |
| Flash point: | 2 °C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Crystals |
| pka | 2.40±0.25(Predicted) |
| color | White |
| Water Solubility | 50 g/100 mL |
| λmax | 223nm(lit.) |
| Merck | 14,3040 |
| BRN | 2453039 |
| CAS DataBase Reference | 1918-00-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzoic acid, 3,6-dichloro-2-methoxy-(1918-00-9) |
| EPA Substance Registry System | Dicamba (1918-00-9) |
Safety information for Dicamba
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Dicamba
Abamectin manufacturer
Gharda Chemicals Ltd
Hemani Industries Limited
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1918-00-9 Dicamba 98%View Details
1918-00-9 Dicamba 98%View Details
1918-00-9 -
 1918-00-9 99%View Details
1918-00-9 99%View Details
1918-00-9 -
 Dicamba >98% (GC) CAS 1918-00-9View Details
Dicamba >98% (GC) CAS 1918-00-9View Details
1918-00-9 -
 Dicamba CAS 1918-00-9View Details
Dicamba CAS 1918-00-9View Details
1918-00-9 -
 Dicamba CAS 1918-00-9View Details
Dicamba CAS 1918-00-9View Details
1918-00-9 -
 Dicamba 1918-00-9 99%View Details
Dicamba 1918-00-9 99%View Details
1918-00-9 -
 Dicamba CAS 1918-00-9View Details
Dicamba CAS 1918-00-9View Details
1918-00-9 -
 Dicamba CAS 1918-00-9View Details
Dicamba CAS 1918-00-9View Details
1918-00-9