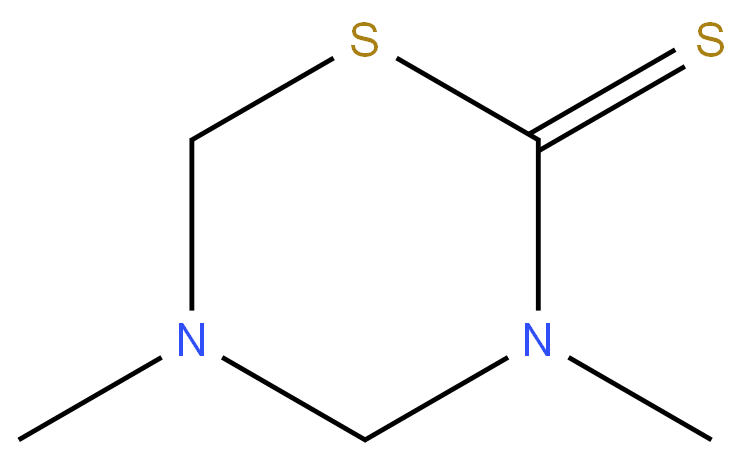
Dazomet
Synonym(s):3,5-Dimethyltetrahydro-1,3,5-thiadiazine-2-thione;Thiadiazine
- CAS NO.:533-74-4
- Empirical Formula: C5H10N2S2
- Molecular Weight: 162.28
- MDL number: MFCD00023809
- EINECS: 208-576-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
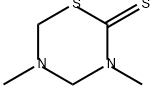
What is Dazomet?
Description
Dazomet is a biocide used to control bacterial and fungal growth in pulp and paper systems, but also in agriculture for soil disinfection. It is contained in Busan 1058, Mylone and Fungicide 974 (Crag). Sensitization, rarely reported, occurred in a paper-mill worker.
Chemical properties
White crystalline solid. Nearly odorless.
The Uses of Dazomet
Dazomet is a as fumigant used for the control of brown root rot disease. Dazomet was shown to be a possible agent in preventing pest incursion in agriculture, gardening and agroforestry.
The Uses of Dazomet
Soil fungicide, nematocide, weed killer; slimicide in paper making.
The Uses of Dazomet
Soil fungicide; nematocide; herbicide; insecticide; soil sterilant.
The Uses of Dazomet
Dazomet is a multi-purpose soil fumigant used for the control of nematodes, soil fun@, soil insects and weed seeds. It is also used as a slimicide in pulp and paper manufacture and as a preservative of adhesives.
What are the applications of Application
Dazomet is a common soil fumigant
Definition
ChEBI: A dithiocarbamic ester that is 1,3,5-thiadiazinane with a thione moiety at position 2 and in which the hydrogens attached to the nitrogens are replaced by methyl groups. A fungicide, herbicide and nematicide, it is used prior to sowing or planting for the ontrol of soil fungi, nematodes, bacteria and germinating weeds, and as fumigant for poultry litter and eggs to control Salmonella. It is a non-ozone-depleting alternative to methyl bromide.
General Description
White crystals or off-white powder. Pungent, acrid odor.
Air & Water Reactions
Insoluble in water. Slowly hydrolyzed.
Reactivity Profile
Dazomet decomposes in water, dilute acids and alcohol.
Fire Hazard
Flash point data for Dazomet are not available; however, Dazomet is probably combustible.
Contact allergens
Dazomet is a biocide used to control bacterial and fungal growth in a pulp and paper system, and also in agriculture for soil disinfection. It is contained in Busan 1058, Mylone, and Fungicide 974 (Crag?). Sensitization, rarely reported, occurred in a paper mill worker.
Safety Profile
Poison bj 7 in ' g estion and intraperitoneal routes. Moderately toxic by skin contact and subcutaneous routes. A severe eye irritant. A mild primary skin irritant and sensitizer. When heated to decomposition it emits very toxic fumes of NOx and SOx
Potential Exposure
Dazomet is a dithiocarbamate insecti- cide, herbicide, fumigant, fungicide, nematicide. It is used as a soil fumigant used against germinating weed seeds, soil insects, nematodes, and soil-borne diseases in forest nursery seed beds, tobacco crops, greenhouse crops, and substrates for potted plants, turf, and ornamentals. It is also used as an antimicrobial agent for slimicide preparations and for adhesives, paper-mill slimicide, paint, and cooling water slimicides.
Environmental Fate
Soil. Soil metabolites include formaldehyde, hydrogen sulfide, methylamine and
methyl(methylaminomethyl)dithiocarbamic acid (Hartley and Kidd, 1987) which further
decomposes to methyl isothiocyanate (Harley and Kidd, 1987; Ashton and Monaco, 1991;
Cremlyn, 1991). The rate of decomposition is dependent upon the soil type, temperature
and humidity (Cremlyn, 1991).
Chemical/Physical. Hydrolyzes in acidic solutions forming carbon disulfide, methylamine and formaldehyde (Hartley and Kidd, 1987; Humburg et al., 1989). These compounds are probably formed following the decomposition of dazomet with alcohol and
Metabolic pathway
Dazomet is a propesticide that decomposes to generate the highly volatile methyl isothiocyanate. The pathways of metabolism of methyl isothiocyanate are described under its own entry. In soil, dazomet degrades mainly via a dithiocarbamic acid which in turn affords methyl isothiocyanate, formaldehyde, hydrogen sulfide and methy lamine.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Degradation
Dazomet is rapidly hydrolysed in acidic media to carbon disulfide, formaldehyde and methylamine (PM). DT50 values for hydrolysis were 85, 490 and 110 hours at pH 5,7 and 9, respectively. It is sensitive to oxygen and to light.
Incompatibilities
Sensitive to moisture and heat. Decomposes on heating above 102 C, producing toxic fumes including nitrogen oxides and sulfur oxides. Dithiocarbamate esters are combustible. They react vio- lently with powerful oxidizers such as calcium hypochlo- rite. Poisonous gases are generated by the thermal decomposition of Dithiocarbamate compounds, including carbon disulfide, oxides of sulfur, oxides of nitrogen, hydrogen sulfide, ammonia, and methylamine. Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids. Flammable gases are generated by the combination of dithiocarbamate with aldehydes, nitrides, and hydrides. Dithiocarbamate are incompatible with acids, peroxides, and acid halides.
Waste Disposal
Do not discharge into drains or sewers. Dispose of waste material as hazardous waste using a licensed disposal contractor to an approved landfill. Consult with environmental regulatory agencies for guid- ance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. A potential candidate for liquid injection incineration at a temperature range of 650℃ to 1600℃ and a residence time 0.1 to 2 seconds. Also, a potential candidate for rotary kiln incineration at a temperature range of 820℃ to 1600℃ and residence times of seconds for liquids and gases, and hours for solids .In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Dazomet
| Melting point: | 104-105°C |
| Boiling point: | 222.3±50.0 °C(Predicted) |
| Density | 1.3 |
| vapor pressure | 3.7 x 10-4 Pa (25 °C) |
| refractive index | 1.5005 (estimate) |
| Flash point: | 156 °C |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), DMSO (Slightly) |
| pka | 4.04±0.20(Predicted) |
| form | neat |
| Water Solubility | <0.1 g/100 mL at 18 ºC |
| Merck | 13,2854 |
| BRN | 116039 |
| CAS DataBase Reference | 533-74-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 2H-1,3,5-thiadiazine-2-thione, tetrahydro-3,5-dimethyl-(533-74-4) |
| EPA Substance Registry System | Dazomet (533-74-4) |
Safety information for Dazomet
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Dazomet
| InChIKey | QAYICIQNSGETAS-UHFFFAOYSA-N |
Dazomet manufacturer
Speciality Organics Pvt. Ltd.
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 533-74-4 99%View Details
533-74-4 99%View Details
533-74-4 -
 Dazomet 533-74-4 98%View Details
Dazomet 533-74-4 98%View Details
533-74-4 -
 Dazomet 98% (GC) CAS 533-74-4View Details
Dazomet 98% (GC) CAS 533-74-4View Details
533-74-4 -
 Dazomet CAS 533-74-4View Details
Dazomet CAS 533-74-4View Details
533-74-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1