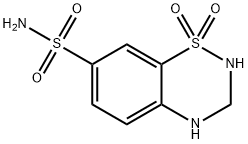
Hydrochlorothiazide
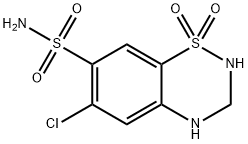
Synonym(s):6-Chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide;6-Chloro-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide;HCTZ;Hydrochlorothiazide
- CAS NO.:58-93-5
- Empirical Formula: C7H8ClN3O4S2
- Molecular Weight: 297.74
- MDL number: MFCD00051765
- EINECS: 200-403-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:12:01
What is Hydrochlorothiazide?
Absorption
An oral dose of hydrochlorothiazide is 65-75% bioavailable, with a Tmax of 1-5 hours, and a Cmax of 70-490ng/mL following doses of 12.5-100mg. When taken with a meal, bioavailability is 10% lower, Cmax is 20% lower, and Tmax increases from 1.6 to 2.9 hours.
Toxicity
The oral LD50 of hydrochlorothiazide is >10g/kg in mice and rats.
Patients experiencing an overdose may present with hypokalemia, hypochloremia, and hyponatremia. Treat patients with symptomatic and supportive treatment including fluids and electrolytes. Vasopressors may be administered to treat hypotension and oxygen may be given for respiratory impairment.
Originator
Hydrodiuril,MSD,US,1959
The Uses of Hydrochlorothiazide
Hydrochlorothiazide is a carbonic anhydrase inhibitor that acts as a diuretic and is one of the most widely used drugs in the family. It has the same indications as chlorothiazide and has a lesser inhibitory effect on carbonic anhydrase, but at the same dose, hydrochlorothiazide is 5-10 times more diuretic than chlorothiazide for sodium ions.
Background
Hydrochlorothiazide is the most commonly prescribed thiazide diuretic. It is indicated to treat edema and hypertension. Hydrochlorothiazide use is common but declining in favour of angiotensin converting enzyme inhibitors. Many combination products are available containing hydrochlorothiazide and angiotensin converting enzyme inhibitors or angiotensin II receptor blockers.
Hydrochlorothiazide was granted FDA approval on 12 February 1959.
Indications
Hydrochlorothiazide is indicated alone or in combination for the management of edema associated with congestive heart failure, hepatic cirrhosis, nephrotic syndrome, acute glomerulonephritis, chronic renal failure, and corticosteroid and estrogen therapy. Hydrochlorothiazide is also indicated alone or in combination for the management of hypertension.
What are the applications of Application
Hydrochlorothiazide is a carbonic anhydrase inhibitor
Definition
ChEBI: A benzothiadiazine that is 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide substituted by a chloro group at position 6 and a sulfonamide at 7. It is diuretic used for the treatment of hypertension and congestive heart failure.
Manufacturing Process
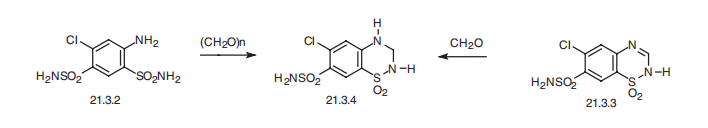
As described in US Patent 3,163,645, a mixture of 2.9 grams of 5-chloro-2,4-
disulfamyl aniline in 15 ml of anhydrous diethyleneglycol dimethyl ether, 0.5
ml of an ethyl acetate solution containing 109.5 grams of hydrogen chloride
per 1,000 ml and 0.33 grams (0.011 mol) of paraformaldehyde is heated to
80° to 90°C and maintained at that temperature for 1 hour. The resulting
mixture is cooled to room temperature and concentrated to one-third of its volume under reduced pressure, diluted with water, then allowed to
crystallize. The product is filtered off and recrystallized from water, to yield
the desired 6-chloro-7-sulfamyl-3,4-dihydro-2H-[1,2,4]-benzothiadiazine-1,1-
dioxide, MP 266° to 268°C, yield 1.4 grams. By replacing paraformaldehyde
by 0.84 gram of 1,1 -dimethoxymethane and proceeding as above, the same
compound is obtained.
As described in US Patent 3,025,292, the desired product may be made by
hydrogenation of chlorothiazide. Three grams of 6-chloro-7-sulfamyl-1,2,4-
benzothiadiazine-1,1-dioxide (chlorothiazide) is suspended in 100 ml of
methanol. Then 1.0 gram of a 5% ruthenium on charcoal catalyst is added,
and the mixture is reduced at room temperature and at an initial hydrogen
pressure of 39 psig. The theoretical amount of hydrogen to form the 3,4-
dihydro derivative is absorbed after a period of about 10 hours.
The reduction mixture then is heated to boiling and filtered hot to remove the
catalyst. The catalyst is washed with a little methanol and the combined
filtrate is concentrated to a volume of about 25 ml by evaporation on a steam
bath. Upon cooling to room temperature, white crystals separate which are
filtered, washed with water, and dried in vacuo at room temperature over
phosphorus pentoxide overnight. The weight of 6-chloro-7-sulfamyl 3,4-
dihydro-1,2,4-benzothiadiazine-1,1-dioxide obtained is 1.26 grams; MP 268.5°
to 270°C. Dilution of the above filtrate with water to a volume of about 125
ml gives a second crop of product having the same melting point and
weighing 1.22 grams, giving a combined yield of 83%. When the product is
mixed with an authentic sample of 6-chloro-7-sulfamyl-3,4-dihydro-1,2,4-
benzothiadiazine-1,1-dioxide, prepared by another method, the melting point
is not depressed.
brand name
Esidrix (Novartis); Hydro-D (Halsey); Hydrodiuril (Merck); Microzide (Watson); Oretic (Abbott); Zide (Solvay Pharmaceuticals).
Reactivity Profile
Strong reducing agents will produce toxic gases ammonia and hydrogen sulfide.
Fire Hazard
Flash point data for Hydrochlorothiazide are not available but Hydrochlorothiazide is probably combustible.
Pharmacokinetics
Hydrochlorothiazide prevents the reabsorption of sodium and water from the distal convoluted tubule, allowing for the increased elimination of water in the urine. Hydrochlorothiazide has a wide therapeutic window as dosing is individualized and can range from 25-100mg. Hydrochlorothiazide should be used with caution in patients with reduced kidney or liver function.
Safety Profile
Poison bp intraperitoneal and intravenous routes. Moderately toxic by ingestion and subcutaneous routes. Human systemic effects by ingestion: sodum level changes, chlorine level changes, acute pulmonary edema, nausea or vomiting. Experimental reproductive effects. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. A duetic. When heated to decomposition it emits very toxic fumes of SOx, Cl-, and NOx.
Synthesis
Hydrochlorothiazide, 1,1-dioxide 6-chloro-3,4-dihydro-2H-1,2,4- benzothiadiazin-7-sulfonamide (21.3.4), is synthesized either by cyclization of 4,6-sulfonamido- 3-chloroaniline (21.3.2) using paraformaldehyde, during which simultaneous reduction of the double bond occurs at position C3¨CC4, or the drug is synthesized by reduction of the same double bond in chlorothiazide (21.3.3) by formaldehyde. This small change in structure increases activity of the drug in comparison with chlorothiazide, and increases its absorbability when used orally.
Veterinary Drugs and Treatments
In veterinary medicine, furosemide has largely supplanted the use of thiazides as a general diuretic (edema treatment). Thiazides are still used for the treatment of systemic hypertension, nephrogenic diabetes insipidus, and to help prevent the recurrence of calcium oxalate uroliths in dogs.
Properties of Hydrochlorothiazide
| Melting point: | 273 °C |
| Boiling point: | 577.0±60.0 °C(Predicted) |
| Density | 1.6761 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | Very slightly soluble in water, soluble in acetone, sparingly soluble in ethanol (96 per cent). It dissolves in dilute solutions of alkali hydroxides |
| form | solid |
| pka | 7.9, 9.2(at 25℃) |
| color | White to Off-White |
| Odor | wh. or pract. wh. cryst. powd., odorless |
| Water Solubility | 722mg/L(25 ºC) |
| λmax | 318nm(H2O)(lit.) |
| Merck | 14,4781 |
| BRN | 625101 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 58-93-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 6-Chloro-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine-1,1-dioxide(58-93-5) |
| IARC | 2B (Vol. 50, 108) 2016 |
| EPA Substance Registry System | Hydrochlorothiazide (58-93-5) |
Safety information for Hydrochlorothiazide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Hydrochlorothiazide
| InChIKey | JZUFKLXOESDKRF-UHFFFAOYSA-N |
Hydrochlorothiazide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Hydrochlorothiazide 99%View Details
Hydrochlorothiazide 99%View Details -
 Hydrochlorothiazide 98%View Details
Hydrochlorothiazide 98%View Details -
 Hydrochlorothiazide 99%View Details
Hydrochlorothiazide 99%View Details -
 Hydrochlorothiazide >98% (HPLC) CAS 58-93-5View Details
Hydrochlorothiazide >98% (HPLC) CAS 58-93-5View Details
58-93-5 -
 Hydrochlorothiazide 98% CAS 58-93-5View Details
Hydrochlorothiazide 98% CAS 58-93-5View Details
58-93-5 -
 Hydrochlorothiazide 95.00% CAS 58-93-5View Details
Hydrochlorothiazide 95.00% CAS 58-93-5View Details
58-93-5 -
 Hydrochlorothiazide IHRSView Details
Hydrochlorothiazide IHRSView Details
58-93-5 -
 Hydrochlorothiazide APIView Details
Hydrochlorothiazide APIView Details
58-93-5
