Buprofezin
- CAS NO.:69327-76-0
- Empirical Formula: C16H23N3OS
- Molecular Weight: 305.44
- MDL number: MFCD01681899
- EINECS: 614-948-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
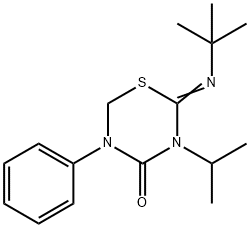
What is Buprofezin?
The Uses of Buprofezin
Buprofezin is a insecticide which works as a chitin synthesis inhibitor. Buprofezin is used to control stubborn rice pests and is effective in controlling green leaf hoppers and white back plant hoppe rs for long durations.
The Uses of Buprofezin
Insecticide.
The Uses of Buprofezin
Buprofezin is a contact and ingested insecticide, active against Homoptera (whiteflies, leafhoppers, scale insects, etc.) in and on citrus, cotton, cucumbers, tomatoes, sweet potatoes, rice, etc.
What are the applications of Application
Buprofezin is an insecticide that acts as a chitin synthesis inhibitor it is effective for long durations
Definition
ChEBI: Buprofezin is a 2-(tert-butylimino)-5-phenyl-3-(propan-2-yl)-1,3,5-thiadiazinan-4-one in which the C=N double bond has Z configuration. It has a role as an insecticide and a member of homopteran inhibitor of chitin biosynthesis.
Hazard
Moderately toxic by ingestion. Low toxic- ity by skin contact.
Agricultural Uses
Insecticide, Acaricide, Insect growth regulator: For insect control in food crops and greenhouse ornamentals.
Trade name
APPLAUD®; NNI-750®
Pharmacology
The foregoing indicates that the modes of action of buprofezin and benzoylureas could be similar or identical. However, one differing biochemical effect of buprofezin is inhibition of prostaglandin biosynthesis (33), a mechanism that has been suggested as responsible for its ovicidal activity. Subsequently, the in vitro and in vivo effects of buprofezin were found to be strongly antagonized by 20- hydroxyecdysone (34), which also affected prostaglandin biosynthesis. Thus, inhibition of both prostaglandin and chitin biosynthesis by buprofezin was prevented by 20- hydroxyecdysone, so that both effects of the insecticide are mediated via an effect on the hormone concentration or its receptor. Consequently, buprofezin seems to inhibit the drop in the 20-hydroxyecdysone titer that triggers epidermal cell proliferation, old cuticle digestion, and new cuticle deposition, but the detailed mechanism of this action has yet to be established.
Metabolic pathway
Buprofezin gradually decomposes in soils under flooded and upland conditions, with half-lives of 104 and 80 days, respectively. After 150 days, five degradation products are identified as 2-tert- butylimino-5-(4-hydroxyphenyl)-3-isopropylperhydro- 1,3,5-thiadiazin-4-one, 3-isopropyl-5-phenylperhydro- 1,3,5-thiadiazin-2,4-dione, 1-tert-butyl-3-ispropyl-5- phenylbiuret, 1-isopropyl-3-phenylurea, and phenylurea. As minor products, 2-tert-butylimino-5- phenylperhydro-1,3,5-thiadiazin-4-one or buprofezin sulfoxide are found in the flooded or in the upland soils. Since neither formation of 14CO2 nor hydroxylation is observed in the sterile soils, buprofezin seems to have undergone complete mineralization in soils under both conditions through biological transformation by soil microorganisms.
Degradation
Buprofezin (1) was degraded under acidic conditions with half-lives
(DT50) f 6-12 days (pH 4), 34 days (pH 6) and 65 days (pH 10) at 40 °C.
Opening of the thiadiazinanone ring appeared to be the primary hydrolytic
degradation pathway, to yield 1-tert-butyl-3-isopropyl-5-phenyl-2-
thiobiuret (2) and N-isopropyl-N-phenylurea (3) as major products.
Buprofezin is stable to aqueous photolysis. The estimated DT50 of
buprofezin in distilled water when exposed to UV light was 39 days. A
more complex photodegradation pathway of buprofezin in methanol was
reported recently (Datta and Walia, 1997) with DT9 values of 4 hours and
15 days under UV and sunlight irradiation, respectively.
Properties of Buprofezin
| Melting point: | 104-106°C |
| Boiling point: | 273°C (12 torr) |
| alpha | 22 º (c=8,6N HCl) |
| Density | 1.18 |
| vapor pressure | 1.25 x l0-3 Pa (25 °C) |
| refractive index | 1.52-1.522 |
| Flash point: | 176-178°C |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | neat |
| pka | 3.02±0.20(Predicted) |
| form | Solid |
| color | White to Pale Beige |
| Water Solubility | 0.9 mg/L at 20 ºC |
| Decomposition | 176-178 ºC |
| BRN | 8324923 |
| CAS DataBase Reference | 69327-76-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 4H-1,3,5-thiadiazin-4-one, 2-((1,1-dimethylethyl)imino)tetrahydro-3-(1-methylethyl)-5-phenyl-(69327-76-0) |
| EPA Substance Registry System | Buprofezin (69327-76-0) |
Safety information for Buprofezin
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
Computed Descriptors for Buprofezin
Buprofezin manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 69327-76-0 Buprofezin 98%View Details
69327-76-0 Buprofezin 98%View Details
69327-76-0 -
 Buprofezin 97.00% CAS 69327-76-0View Details
Buprofezin 97.00% CAS 69327-76-0View Details
69327-76-0 -
 Buprofezin Technical 98% Min.View Details
Buprofezin Technical 98% Min.View Details
69327-76-0 -
 Ambazin (Buprofezin 25%SC), Hdpe BottleView Details
Ambazin (Buprofezin 25%SC), Hdpe BottleView Details
69327-76-0 -
 Buprofezin 25 Sc InsecticideView Details
Buprofezin 25 Sc InsecticideView Details
69327-76-0 -
 Liquid Buprofezin Technical, 50 LView Details
Liquid Buprofezin Technical, 50 LView Details
69327-76-0 -
 Liquid Buprofezin 25 SC Insecticide, 1 LView Details
Liquid Buprofezin 25 SC Insecticide, 1 LView Details
69327-76-0 -
 Buprofezin 25% SC Chemicals, Drum, 200 LView Details
Buprofezin 25% SC Chemicals, Drum, 200 LView Details
69327-76-0
