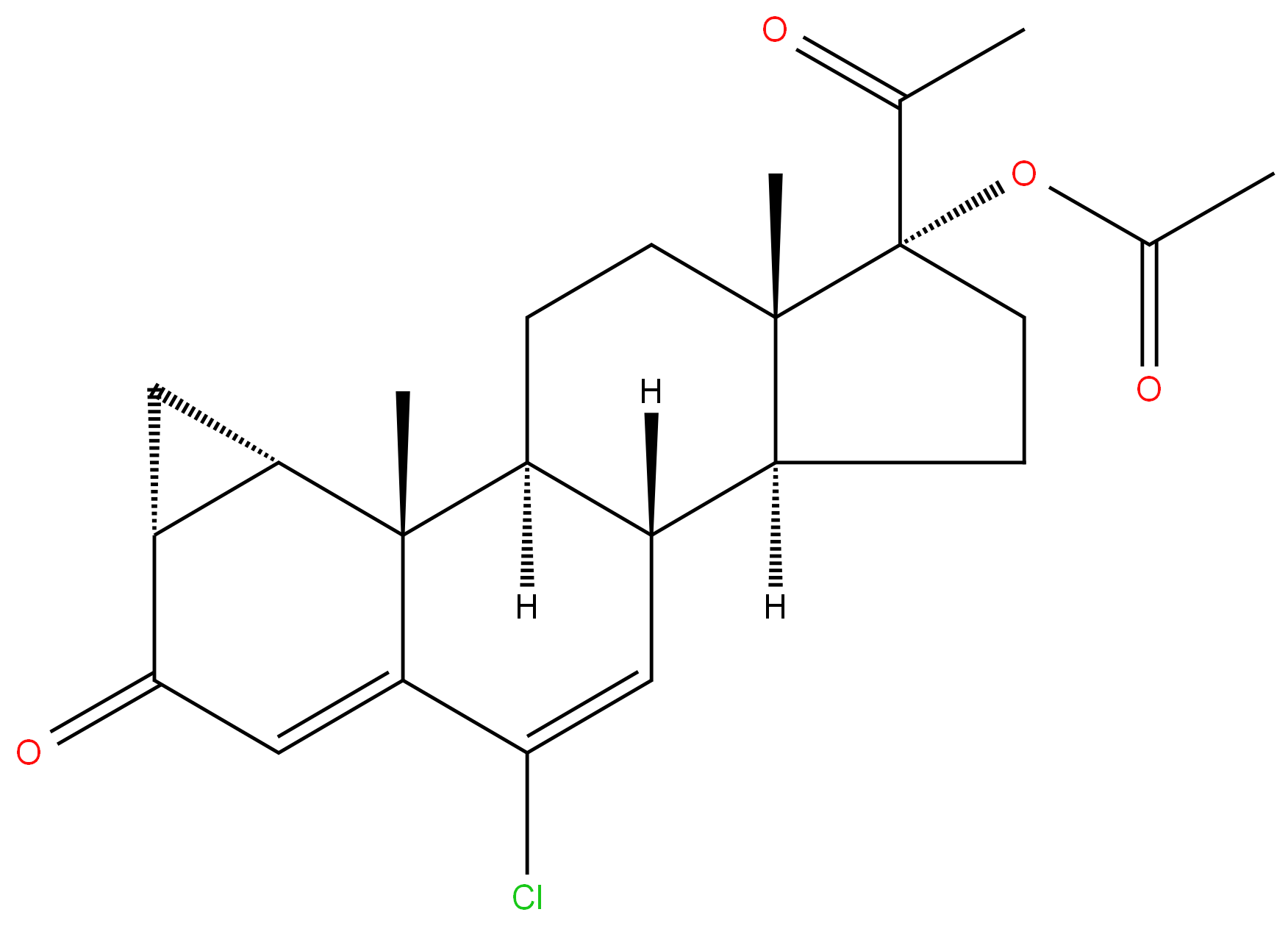
Cyproterone acetate
Synonym(s):6-Chloro-1β,2β-dihydro-17-hydroxy-3′H-cyclopropa(1,2)-pregna-1,4,6-triene-3,20-dione acetate;Cyproterone acetate
- CAS NO.:427-51-0
- Empirical Formula: C24H29ClO4
- Molecular Weight: 416.94
- MDL number: MFCD00864671
- EINECS: 207-048-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
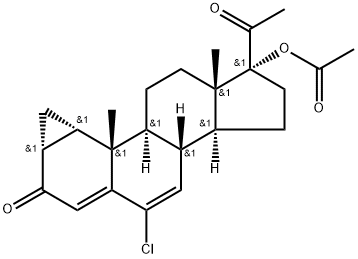
What is Cyproterone acetate?
Absorption
Completely absorbed following oral administration.
Description
Cyproterone acetate (CPA) is an androgen receptor antagonist. It binds to human androgen receptors (Ki = 14 nM) and inhibits dihydrotestosterone-induced androgen receptor activation in CV-1 cells (IC50 = 26 nM). CPA (30 mg/kg) decreases ventral prostate weight in castrated immature rats. It also suppresses accessory sexual glands and fertility in adult male rats when administered at a dose of 10 mg/kg. CPA (0.3 μM) also induces apoptosis in primary adult female rat hepatocytes. Formulations containing cyproterone acetate have been used in the treatment of androgenization in females.
Chemical properties
Crystalline Solid
Originator
Androcur ,Schering,W. Germany,1973
The Uses of Cyproterone acetate
The free alcohol is an anti-androgen; cyproterone acetate is both an anti-androgen and a progestogen. Combined with estrogen it is used in the treatment of acne.
The Uses of Cyproterone acetate
Cyproterone acetate (CPA) has been shown to exert gestagenic and glucocorticoid effects. Because it inhibits the negative diencephalic pituitary testicular feedback system, it lowers luteinizing hormone (LH) and testosterone production and plasma levels. cyproterone acetate is both an anti-androgen and a progestogen. CPA is used in combined birth control pills, in the treatment of androgen-dependent skin and hair conditions such as acne (Combined with estrogen), seborrhea, excessive hair growth, and scalp hair loss, high androgen levels, in transgender hormone therapy, to treat prostate cancer, to reduce sex drive in sex offenders or men with paraphilias or hypersexuality, to treat early puberty, and for other uses. It is used both at low doses and at higher doses.
Background
An anti-androgen that, in the form of its acetate (cyproterone acetate), also has progestational properties. It is used in the treatment of hypersexuality in males, as a palliative in prostatic carcinoma, and, in combination with estrogen, for the therapy of severe acne and hirsutism in females.
Indications
For the palliative treatment of patients with advanced prostatic carcinoma.
What are the applications of Application
Cyproterone Acetate is a compound that induces apoptosis in hepatocytes
Indications
Cyproterone acetate is a progestational antiandrogen that blocks androgen receptor binding and suppresses androgen-sensitive tissues. It is available in a topical form in Europe for the treatment of hirsutism.
Definition
ChEBI: Cyproterone acetate is a steroid ester resulting from the formal condensation of the carboxy group of acetic acid with the 17-hydroxy group of cyproterone. It is an antiandrogenic drug which has recently been recognized to promote the occurrence and growth of intracranial meningiomas. It has a role as an androgen antagonist, a progestin and a geroprotector. It is a 20-oxo steroid, a 3-oxo-Delta(4) steroid, a chlorinated steroid, a steroid ester and an acetate ester. It is functionally related to a cyproterone.
Manufacturing Process
2.34 g of 1,2α-methylene-δ4,6-pregnadiene-17α-ol-3,20-dione-17-acetate are
dissolved in 18.25 cc of ethylene chloride which contains 844 rng of
perbenzoic acid. The solution is stored for 16 hours at +5°C and 7 hours at
room temperature. It is then diluted with methylene chloride and, with
aqueous ferrous sulfate solution, sodium bicarbonate solution and with water
washed until neutral.
The organic phase is dried over sodium sulfate and then concentrated to
dryness. 1.62 g of the thus obtained crude 1,2α-methylene-6,7α-oxido-δ4-
pregnene-17α-ol-3,20-dione-17-acetate are dissolved in 109 cc of glacial
acetic acid. This solution is then saturated at room temperature with hydrogen
chloride gas and stored for 20 hours, It is then diluted with methylene
chloride and washed with water until neutral.
The organic phase is dried over sodium sulfate and then concentrated to
dryness. The thus obtained crude 6-chloro-1α-chloromethyl-δ4,6-pregnadiene17α-ol-3,20-dione-17-acetate is heated to boiling in 20 cc of collidine for 20
minutes under nitrogen. After dilution with ether it is washed with 4 N
hydrochloric acid and washed with water until neutral.
After drying over sodium sulfate and concentration to vacuum the remaining
residue is subjected to chromatography over silica gel. Using a benzene-ethyl
acetate mixture (19:1) there is eluated 900 mg of 6-chloro-1,2α-methyleneδ4,6-pregnadiene-17α-ol-3,20-dione-17-acetate, which upon recrystallization
from isopropyl ether melts at 200° to 201°C.
Therapeutic Function
Antiandrogen
Mechanism of action
The mechanism of action of Cyproterone acetate at the molecular level has never been described conclusively. Studies have found that in rat ventral prostate nuclei, CPA interferes with androgen binding to the androgen receptor. It is possible that CPA interferes with the translocation of the androgen receptor from the cytoplasm to the nuclear compartment. At the transcription level, the effects of CPA also include a reduction of androgen-dependent cellular functions, such as a reduction of acid phosphatase, prostate-specific androgen, and androgen-dependent enzymes such as 5-a-reductase[1].
Pharmacokinetics
Cyproterone is an antiandrogen. It suppresses the actions of testosterone (and its metabolite dihydrotestosterone) on tissues. It acts by blocking androgen receptors which prevents androgens from binding to them and suppresses luteinizing hormone (which in turn reduces testosterone levels).
Side Effects
Vbreast tenderness and swelling. Tiredness and weakness (fatigue). Depression or mood changes. Hot flushes or sweats. Swelling in parts of the body (fluid build up). Weight changes. Liver changes (you will have regular blood tests to check for this). Shortness of breath.
Metabolism
Primarily hepatic. Cyproterone acetate is metabolized by the CYP3A4 enzyme, forming the active metabolite 15beta-hydroxycyproterone acetate, which retains its antiandrogen activity, but has reduced progestational activity.
Metabolism
Cyproterone is metabolised by various pathways including hydroxylation and conjugation; about 35% of a dose is excreted in urine, the remainder being excreted in the bile. The principal metabolite, 15β-hydroxycyproterone, has anti-androgenic activity
References
[1] Schr?der F, et al. Cyproterone Acetate-Mechanism of Action and Clinical Effectiveness in Prostate Cancer Treatment. Cancer, 1993; 72: 3810-3815.
Properties of Cyproterone acetate
| Melting point: | 200-201°C |
| Boiling point: | 528.8°C (rough estimate) |
| Density | 1.0677 (rough estimate) |
| refractive index | 1.4429 (estimate) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Practically insoluble in water, very soluble in methylene chloride, freely soluble in acetone, soluble in methanol, sparingly soluble in anhydrous ethanol. |
| form | neat |
| form | Solid |
| color | White to Off-White |
| λmax | 281nm(MeOH)(lit.) |
| Merck | 14,2774 |
| EPA Substance Registry System | 3'H-Cyclopropa[1,2]pregna-1,4,6-triene-3,20-dione, 17-(acetyloxy)-6-chloro-1,2-dihydro-, (1.beta.,2.beta.)- (427-51-0) |
Safety information for Cyproterone acetate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Cyproterone acetate
| InChIKey | UWFYSQMTEOIJJG-KIXORDEXNA-N |
| SMILES | C[C@@]12C(=CC(=O)[C@@H]3C[C@H]13)C(Cl)=C[C@@]1([H])[C@]3([H])CC[C@@](C(=O)C)(OC(=O)C)[C@@]3(C)CC[C@]21[H] |&1:1,6,8,12,14,18,26,30,r| |
Cyproterone acetate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 CYPROTERONE ACETATE 99%View Details
CYPROTERONE ACETATE 99%View Details -
 427-51-0 98%View Details
427-51-0 98%View Details
427-51-0 -
 Cyproterone Acetate CAS 427-51-0View Details
Cyproterone Acetate CAS 427-51-0View Details
427-51-0 -
 Cyproterone acetate 97% (HPLC) CAS 427-51-0View Details
Cyproterone acetate 97% (HPLC) CAS 427-51-0View Details
427-51-0 -
 Cyproterone acetate CAS 427-51-0View Details
Cyproterone acetate CAS 427-51-0View Details
427-51-0 -
 Cyproterone acetate CAS 427-51-0View Details
Cyproterone acetate CAS 427-51-0View Details
427-51-0 -
 Cyproterone acetate CAS 427-51-0View Details
Cyproterone acetate CAS 427-51-0View Details
427-51-0 -
 Cyproterone acetate CAS 427-51-0View Details
Cyproterone acetate CAS 427-51-0View Details
427-51-0
