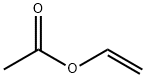
POLY(VINYL ACETATE)
Synonym(s):Poly(vinyl acetate);Poly(vinyl acetate) dispersion 30 %;Poly(vinyl acetate) stabilized with polyvinylpyrrolidone and sodium lauryl sulfate;Polyvinyl acetate;Polyvinyl acetate stabilized with povidone and sodium lauryl sulfate
- CAS NO.:9003-20-7
- Empirical Formula: (C4H6O2)x
- Molecular Weight: 86.09
- MDL number: MFCD00084457
- EINECS: 203-545-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:40

What is POLY(VINYL ACETATE)?
Chemical properties
transparent pellets or white powder
Chemical properties
The degree of polymerization of polyvinyl acetate typically is 100 to 5000. The ester groups of the polyvinyl acetate are sensitive to base hydrolysis and will slowly convert PVAc into polyvinyl alcohol and acetic acid.
Under alkaline conditions, boron compounds such as boric acid or borax cause the polymer to cross-link, forming tackifying precipitates or slime.
History
Polyvinyl acetate was discovered in Germany in 1912 by Fritz Klatte.
The monomer, vinyl acetate, was first produced on an industrial scale by addition of acetic acid to acetylene with a mercury(I) salt[3] but it is now primarily made by palladium catalyzed oxidative addition of acetic acid to ethylene.
The Uses of POLY(VINYL ACETATE)
drug delivery, hemodynamics, wound dressing, coatings
The Uses of POLY(VINYL ACETATE)
As an emulsion in water, aPVAc emulsions are used as adhesives for porous materials, particularly for wood, paper, and cloth, and as a consolidant for porous building stone, in particular sandstone . Uses :
As wood glue PVAc is known as "white glue" and the yellow "carpenter's glue" or PVA glue.
As paper adhesive during paper packaging converting
In bookbinding and book arts, due to its flexible strong bond and non-acidic nature (unlike many other polymers). The use of PVAC on the Archimedes Palimpsest during the 20th century greatly hindered the task of disbinding the book and preserving and imaging the pages in the early 21st century, in part because the glue was stronger than the parchment it held together.
The stiff homopolymer PVAc, but mostly the more soft copolymer a combination of vinyl acetate and ethylene, vinyl acetate ethylene (VAE), is used also in paper coatings, paint and other industrial coatings, as binder in nonwovens in glass fibers. sanitary napkins, filter paper and in textile finishing
PVAc can also be used as coating to protect cheese from fungi and humidity.
Polyvinyl acetate is also the raw material to make other polymers like : Polyvinyl alcohol -[HOCHCH2]-: Polyvinyl acetate is partially or completely hydrolysed to give polyvinyl alcohol. This reversible saponification and esterification reaction was a strong hint for Hermann Staudinger in the formulation of his theory of macro molecules.
Polyvinyl acetate phthalate (PVAP): Polyvinyl acetate is partially hydrolyzed and then esterified with phthalic acid.
The Uses of POLY(VINYL ACETATE)
Polyvinyl acetate is used as an adhesive for porous materials such as wood, paper, cloth and in handicrafts. It also finds application as a primer for drywall, as wallpaper adhesive, as the film-forming ingredient in water-based (latex) paints and as an envelope adhesive. It is used as a raw material for the preparation of other polymers like polyvinyl alcohol and polyvinyl acetate phthalate (PVAP). It plays an important role in the lamination of metal foils.
Definition
ChEBI: A polymer composed of repeating acetoxyethylene units.
Preparation
PVAc is a vinyl polymer. Polyvinyl acetate is prepared by polymerization of vinyl acetate monomer (free radical vinyl polymerization of the monomer vinyl acetate).
Production Methods
Polyvinyl acetate is derived from the polymerization of vinyl acetate; the catalysts used in polymerization may include hydrogen peroxide, peroxy sulfates, or various redox combinations. The polymerization process is described as being carried out by charging all ingredients to the reactor, heating to reflux, and stirring until the reaction is complete. Typically, only a part of the monomer and catalyst is initially charged; the remainder is added during the course of the reaction.
General Description
Poly(vinyl acetate) [PVAc] is a biocompatible and biodegradable polymer as it has hydrolyzable groups in the side chain,also it is non-toxic and non-carcinogenic.Owing to its biologically friendly nature , it is used in various biomedical application such as artificial organ implant, contact lens,cardiovascular devices and cartilage skin. It is also used in wound dressing and various drug-delivery applications. It has been accepted as reference standard for universal calibration in gel permeation chromatography.
Hazard
Questionable carcinogen.
Industrial uses
Polyvinyl acetate is a leathery, colorless thermoplasticmaterial that softens at relatively lowtemperatures and that is relatively stable to lightand oxygen. The polymers are clear and noncrystalline.The chief applications of polyvinylacetate are as adhesives and binders for waterbasedor emulsion paints.
Vinyl acetate is conveniently prepared bythe reaction of acetylene with acetic acid.
Safety Profile
Very low toxicity by ingestion. Questionable carcinogen. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS
Purification Methods
Precipitate it from acetone by addition of n-hexane.
Properties of POLY(VINYL ACETATE)
| Melting point: | 60°C |
| Boiling point: | 70-150 °C |
| Density | 1.18 g/mL at 25 °C |
| refractive index | n |
| Flash point: | >100℃ |
| storage temp. | 2-8°C |
| solubility | ketones, ethers and aromatic hydrocarbons: soluble |
| form | pellets |
| color | Clear |
| PH | 3.0-5.5 |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong bases. Combustible. |
| IARC | 3 (Vol. 19, Sup 7) 1987 |
| EPA Substance Registry System | Polyvinyl acetate (9003-20-7) |
Safety information for POLY(VINYL ACETATE)
Computed Descriptors for POLY(VINYL ACETATE)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Polyvinyl acetate, very fine crystalline beads CAS 9003-20-7View Details
Polyvinyl acetate, very fine crystalline beads CAS 9003-20-7View Details
9003-20-7 -
 Poly(vinyl acetate), average Mw ~140,000 CAS 9003-20-7View Details
Poly(vinyl acetate), average Mw ~140,000 CAS 9003-20-7View Details
9003-20-7 -
 Polyvinyl acetate, crystalline beads CAS 9003-20-7View Details
Polyvinyl acetate, crystalline beads CAS 9003-20-7View Details
9003-20-7 -
 Polyvinyl acetate, granulated lumps CAS 9003-20-7View Details
Polyvinyl acetate, granulated lumps CAS 9003-20-7View Details
9003-20-7 -
 Poly(vinyl acetate), average Mw ~500,000 CAS 9003-20-7View Details
Poly(vinyl acetate), average Mw ~500,000 CAS 9003-20-7View Details
9003-20-7 -
 Polyvinyl acetate dispersion CASView Details
Polyvinyl acetate dispersion CASView Details -
 Laboratory Polyvinyl Acetate (granulars) ALPHAView Details
Laboratory Polyvinyl Acetate (granulars) ALPHAView Details
9003-20-7 -
 POLYVINYL ACETATE : (9003-20-7)View Details
POLYVINYL ACETATE : (9003-20-7)View Details
9003-20-7
