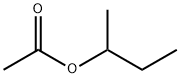
Isopropyl acetate
Synonym(s):Acetic acid isopropyl ester;Degassed and low oxygen isopropyl acetate;IPrOAc;Isopropyl acetate;Isopropyl acetate ZerO2
- CAS NO.:108-21-4
- Empirical Formula: C5H10O2
- Molecular Weight: 102.13
- MDL number: MFCD00008877
- EINECS: 203-561-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:59
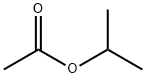
What is Isopropyl acetate ?
Chemical properties
Isopropyl acetate is a colourless liquid with an intense, fruity odor. On dilution, it has a sweet apple-like flavor. Miscible with most organic solvents such as alcohols, ketones and ethers. 2.9% (w/w) soluble in water at 20°C. Synthesized by direct acetylation of isopropyl alcohol in the presence of various catalysts: concentrated H2S04, diethyl sulfate, chlorosulfonic acid, and boron trifluoride.
Physical properties
Clear, colorless liquid with an aromatic odor. Experimentally determined detection and recognition odor threshold concentrations were 2.1 mg/m3 (500 ppbv) and 3.8 mg/m3 (910 ppbv), respectively (Hellman and Small, 1974).
Occurrence
Reported found in pineapple, pear, cocoa, apple, banana, black currants, grapes, melons, strawberry, cheddar cheese, beer, white wine, red wine, cocoa, honey, soybean, yellow passion fruit, beans, plum brandy and nectarines
The Uses of Isopropyl acetate
Isopropyl acetate is used as a solvent for nitrocellulose, plastics, oils, and fats, and as a flavoring agent. Isopropyl Acetate is a widely used chemical solvent in organic and industrial syntheses. Also used in the dissolution of gallstones. Environmental contaminants; Food contaminants.
The Uses of Isopropyl acetate

SOCl2 (20 mL) was added to the SM (2.50 g, 8.47 mmol) and the mixture was refluxed for 16 h. The volatiles were removed in vacuo and the mixture was azeotroped with toluene (3 x 50 mL) to give a gum. To the crude mixture was added 2-methyl tetrahydrofuran (20 mL), followed by TEA (2.36 mL, 17 mmol) and the amine (907 mg, 10.2 mmol). The mixture was stirred at RT for 2 h, quenched with sat aq NaHCO3 (20 mL), and extracted with EtOAc (2 x 20 mL). The combined organics were washed with 10% aq citric acid (20 mL) and concentrated in vacuo to give a gum. The crude was triturated in ether to provide to product as a white solid. [2.32 g, 75%]
Definition
ChEBI: Isopropyl acetate is a branched-chain saturated fatty acid anion that is the conjugate base of isovaleric acid; reported to improve ruminal fermentation and feed digestion in cattle.
Preparation
Isopropyl acetate is prepared from propylene and anhydrous acetic acid in the presence of a catalyst . It may also be produced by direct acetylation of isopropyl alcohol in the presence of various catalysts; concentrated H2SO4, diethyl sulfate, chlorosulfonic acid and boron trifluoride.
What are the applications of Application
Isopropyl acetate is a solvent in chemical industry, especially for cellulose, plastics, waxes, resins, gums, paints, oil and fats. and also as flavoring agent. It is an active component of perfumes and printing inks. It is also employed as an extractant for the preparation of antibiotics, vitamins and hormones.
Aroma threshold values
Detection; 1.7 to 4.4 ppm
Taste threshold values
Taste characteristics at 60 ppm: ethereal, tutti-frutti, with a fruity apple and banana nuance
Synthesis Reference(s)
The Journal of Organic Chemistry, 39, p. 3728, 1974 DOI: 10.1021/jo00939a026
General Description
Isopropyl acetate appears as a clear colorless liquid. Flash point 40°F. Vapors are heavier than air. Contact with the material may irritate skin, eyes or mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Used as a solvent.
Air & Water Reactions
Highly flammable. Less dense than water and slightly soluble in water.
Reactivity Profile
Isopropyl acetate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Isopropyl acetate can react vigorously with nitrates, strong oxidizers, strong alkalis and strong acids. Isopropyl acetate may also attack some forms of rubber, plastics and coatings. .
Health Hazard
Isopropyl acetate is an irritant to the eyes,nose, and throat. Liquid irritates eyes but causes no serious injury; may cause dermatitis; no serious effects if swallowed. The acute toxicity in laboratory animals was low. Exposure to highconcentrations in air or ingestion can produce narcotic effects. A 4-hour exposure to aconcentration of 32,000 ppm in air was fatalto rats (ACGIH 1986). The oral LD50 valuein rats is in the range 6000 mg/kg.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Highly flammable
Source
Identified among 139 volatile compounds identified in cantaloupe (Cucumis melo var. reticulates cv. Sol Real) using an automated rapid headspace solid phase microextraction method (Beaulieu and Grimm, 2001).
Environmental Fate
Chemical/Physical. Hydrolyzes in water forming isopropyl alcohol and acetic acid (Morrison
and Boyd, 1971). The estimated hydrolysis half-life at 25 °C and pH 7 is 8.4 yr (Mabey and Mill,
1978).
At an influent concentration of 1,000 mg/L, treatment with GAC resulted in an effluent
concentration of 319 mg/L. The adsorbability of the carbon used was 137 mg/g carbon (Guisti et
al., 1974).
Purification Methods
Wash the acetate with 50% aqueous K2CO3 (to remove acid), then with saturated aqueous CaCl2 (to remove any alcohol). Dry it with CaCl2 and fractionally distil it. [Beilstein 2 IV 141.]
Properties of Isopropyl acetate
| Melting point: | -73 °C |
| Boiling point: | 88.8 °C |
| Density | 0.872 g/mL at 25 °C(lit.) |
| vapor density | 3.5 (vs air) |
| vapor pressure | 47 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 2926 | ISOPROPYL ACETATE |
| Flash point: | 62 °F |
| storage temp. | Store below +30°C. |
| solubility | 1 M HCl: soluble50mg/mL, clear to slightly hazy, colorless |
| form | Liquid |
| appearance | Colorless liquid |
| color | Clear colorless |
| Odor | Pleasant, fruity; nonresidual. |
| explosive limit | 1.8%, 37°F |
| Odor Threshold | 0.16ppm |
| Water Solubility | 2.90 g/100 mL |
| FreezingPoint | -73.4℃ |
| Sensitive | Moisture Sensitive |
| Merck | 14,5205 |
| JECFA Number | 305 |
| BRN | 1740761 |
| Exposure limits | TLV-TWA 250 ppm (~950 mg/m3)
(ACGIH, MSHA, and OSHA); TLV-STEL
310 ppm (~1185 mg/m3) (ACGIH); IDLH
16,000 ppm (NIOSH). |
| Stability: | Stable. Flammable - note low flash point. Incompatible with strong oxidizing agents, strong acids, nitrates, alkali metals. May attack some plastics and rubber. |
| CAS DataBase Reference | 108-21-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, 1-methylethyl ester(108-21-4) |
| EPA Substance Registry System | Isopropyl acetate (108-21-4) |
Safety information for Isopropyl acetate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for Isopropyl acetate
Isopropyl acetate manufacturer
JSK Chemicals
VJ CHEMTRADE PVT LTD
RAIGAD CHEMICALS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Isopropyl Acetate at Best PriceView Details
Isopropyl Acetate at Best PriceView Details
108-21-4 -
 Iso Propyl Acetate 99%View Details
Iso Propyl Acetate 99%View Details -
 Isopropyl acetate 99%View Details
Isopropyl acetate 99%View Details -
 Isopropyl acetate CAS 108-21-4View Details
Isopropyl acetate CAS 108-21-4View Details
108-21-4 -
 Iso-Propyl Acetate CAS 108-21-4View Details
Iso-Propyl Acetate CAS 108-21-4View Details
108-21-4 -
 Isopropyl acetate CAS 108-21-4View Details
Isopropyl acetate CAS 108-21-4View Details
108-21-4 -
 Isopropyl Acetate CASView Details
Isopropyl Acetate CASView Details -
 Isopropyl Acetate ChemicalsView Details
Isopropyl Acetate ChemicalsView Details
108-21-4
