427-51-0
Product Name:
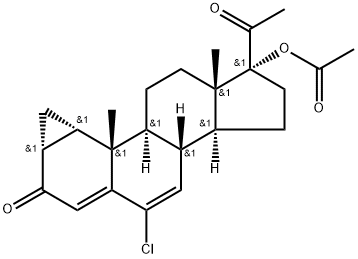
Cyproterone acetate
Formula:
C24H29ClO4
Synonyms:
6-Chloro-1β,2β-dihydro-17-hydroxy-3′H-cyclopropa(1,2)-pregna-1,4,6-triene-3,20-dione acetate;Cyproterone acetate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | Crystals from diisopropyl ether |
|---|---|
| Melting Point | 200-201 |
| Solubility | Very soluble in dichloromethane and acetone; soluble in methanol; sparingly soluble in ethanol. |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Optical Rotation | Specific rotation = +152 deg to +157 deg at 20 °C |
| Decomposition | When heated to decomposition it emits toxic fumes of /hydrogen chloride/. |
| Collision Cross Section | 199.4 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
| Other Experimental Properties | Crystals from acetate, mp 237.5-240 °C /Cyproterone/ |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 416.9 g/mol |
|---|---|
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 416.1754371 g/mol |
| Monoisotopic Mass | 416.1754371 g/mol |
| Topological Polar Surface Area | 60.4 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 903 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Cyproterone acetate is a steroid ester resulting from the formal condensation of the carboxy group of acetic acid with the 17-hydroxy group of cyproterone. It is an antiandrogenic drug which has recently been recognized to promote the occurrence and growth of intracranial meningiomas. It has a role as an androgen antagonist, a progestin and a geroprotector. It is a 20-oxo steroid, a 3-oxo-Delta(4) steroid, a chlorinated steroid, a steroid ester and an acetate ester. It is functionally related to a cyproterone.
