Cupric cyanide
- CAS NO.:14763-77-0
- Empirical Formula: C2CuN2
- Molecular Weight: 115.5808
- MDL number: MFCD01745912
- EINECS: 238-826-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
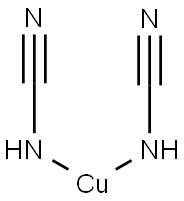
What is Cupric cyanide?
Description
Cuprous cyanide is a white crystalline substance. Cupric cyanide, Cu(CN)2 is a yellowish-green powder which decomposes on heating. Molecularweight =89.56 (cuprous); 115.55 (cupric); Freezing/Melting point =473℃ (in nitrogen) (cuprous cyanide).Hazard Identification (based on NFPA-704 M RatingSystem): Health 3, Flammability 0, Reactivity 0. Insolublein water.
Chemical properties
Cupric cyanide is a yellowish-green powder which decomposes on heating.
Hazard
Toxic.
Safety Profile
Poison by intraperitoneal route. See also CYANIDE and COPPER COMPOUNDS. Incompatible with magnesium. When heated to decomposition it emits toxic fumes of NOx and CN-.
Potential Exposure
Copper cyanide is used in electroplating copper on iron; and as an insecticide and a catalyst.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelyhis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medicalattention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with coppercyanide you should be trained on its proper handling andstorage. Copper cyanide must be stored to avoid contactwith chemically active metals (such as potassium, sodium,magnesium, and zinc) since violent reactions occur. Storein tightly closed containers in a cool, well-ventilated areaaway from acetylene gas.
Shipping
Copper cyanide must carry a “POISONOUS/TOXIC MATERIALS” label. It is classified by DOT inHazard Class 6.1 and Packing Group II.
Incompatibilities
Contact with heat, strong acids (HCl, H2SO4, HNO3) forms deadly hydrogen cyanide gas. May release hydrogen cyanide on contact with moisture. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acetylene gas, and chemically active metals, such as potassium, sodium, magnesium, and zinc.
Waste Disposal
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill. Copper-containing wastes can be concentrated to the point where copper can be electrolytically removed and reclaimed. If recovery is not feasible, the copper can be precipitated by alkali; the cyanide destroyed by alkaline oxidation yielding a sludge which can be sent to a chemical waste landfill. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Cupric cyanide
| Melting point: | decomposes [CRC10] |
| Boiling point: | 41°C (estimate) |
| solubility | insoluble in H2O; soluble in acid solutions, alkaline solutions |
| form | green powder |
| color | green |
| Water Solubility | insoluble H2O; soluble acids and alkalies [HAW93] |
| EPA Substance Registry System | Copper cyanide (Cu(CN)2) (14763-77-0) |
Safety information for Cupric cyanide
Computed Descriptors for Cupric cyanide
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
