NICKEL CYANIDE
- CAS NO.:557-19-7
- Empirical Formula: C2N2Ni
- Molecular Weight: 110.73
- MDL number: MFCD00049490
- EINECS: 209-160-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 15:14:32
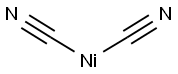
What is NICKEL CYANIDE?
Chemical properties
green powder
Chemical properties
Nickel cyanide is a yellowish-brown plates or powder that may change to a green color by absorbing moisture. Odor is a weak almond odor like cyanide.
Physical properties
The tetrahydrate, Ni(CN)2?4H2O constitutes apple green plates or powder; loses water of crystallization on heating at 200°C; decomposes on further heating; insoluble in water; slightly soluble in dilute acids; soluble in potassium cyanide solution and in ammonia, caustic soda, caustic potash and other bases.
The Uses of NICKEL CYANIDE
Metallurgy, electroplating.
Preparation
Nickel cyanide is prepared by treating a soluble nickel salt, such as nickel chloride or nickel sulfate, with potassium cyanide solution: Ni2+ + 2CNˉ → Ni(CN)2
The product is a tetrahydrate, Ni(CN)2?4H2O, which on heating at 200°C yields yellow-brown anhydrous salt, Ni(CN)2.
Reactions
The most interesting reaction of nickel(II) cyanide is the formation of clathrate compounds in the presence of ammonia. When a solution of Ni(CN)2 in aqueous ammonia is shaken with benzene, a pale violet precipitate of the benzene clathrate Ni(CN)2?NH3?C6H6 is obtained. In this compound the Ni and CN groups form layers with ammonia molecules bonded above and below the planes of the layers on alternate nickel atoms. Thus half the nickel atoms are octahedrally surrounded by nitrogen (Ni-N=2.15 ?, N1-NH3 = 2.06 à) and half are planar 4-coordinated by carbon (Ni-C=1.76?). The benzene molecules occupy the holes (cages) formed between the layers. The average magnetic moment per nickel atom is 2.2 BM, and it has thus been established that the 4- coordinate nickel atoms are diamagnetic while the 6-coordinate atoms are paramagnetic with two unpaired electron spins.
General Description
NICKEL CYANIDE is an apple-green powder or a green crystalline solid. Insoluble in water. Toxic by inhalation and by ingestion. Carcinogenic. Produces toxic oxides of nitrogen in fires.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
NICKEL CYANIDE is thermally unstable and easily oxidized. Weakly basic. Liberates flammable and lethally poisonous hydrogen cyanide gas on contact with acids or acid fumes. Undergoes violent reactions with fluorine, hypochlorites, nitric acid, nitrates, nitrites. Undergoes an explosive reaction if melted with nitrites or chlorates (at about 450°C). Emits highly toxic cyanide fumes when heated to decomposition. [Lewis, 3rd ed., 1993, p. 360, 912]. Undergoes an incandescent reaction if heated with magnesium [Mellor, 1940, vol. 4, p. 271].
Hazard
Highly toxic.
Health Hazard
DUST: POISONOUS IF INHALED. Irritating to eyes, nose and throat. SOLID: POISONOUS IF SWALLOWED. Irritating to skin and eyes.
Fire Hazard
Not flammable.
Safety Profile
Confirmed human carcinogen. A poison. Incandescent reaction when heated with magnesium. When heated to decomposition it emits very toxic fumes of CN-. See also CYANIDE and NICI(EL COMPOUNDS.
Potential Exposure
Nickel cyanide is used in metallurgy, electroplating and making other chemicals.
Shipping
UN1653 Nickel cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. and equipped in OSHA 1910.156. The only respirators recommended for fire fighting are SCBAs that have full facepieces and are operated in a pressure-demand or other positive-pressure mode.
Incompatibilities
Nnickel cyanide is thermally unstable and easily oxidized. Weakly basic. Liberates flammable and lethally poisonous hydrogen cyanide gas on contact with acids or acid fumes. Undergoes violent reactions with fluorine, hypochlorites, nitric acid, nitrates, nitrites. Undergoes an explosive reaction if melted with nitrites or chlorates (at about 450 C). Violent reaction with magnesium. Keep away from acids, active metals, heat, or CO2; contact can cause release of toxic cyanide gas.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of NICKEL CYANIDE
| Melting point: | 200°C -4H₂O |
| Density | 2.393 g/cm3 |
| form | solid |
| Merck | 14,6506 |
| Stability: | Stable, but reacts violently with magnesium. |
| EPA Substance Registry System | Nickel(II) cyanide (557-19-7) |
Safety information for NICKEL CYANIDE
Computed Descriptors for NICKEL CYANIDE
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4