Zinc selenide
Synonym(s):ZN516010
- CAS NO.:1315-09-9
- Empirical Formula: SeZn
- Molecular Weight: 144.35
- MDL number: MFCD00011296
- EINECS: 215-259-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
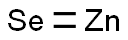
What is Zinc selenide?
Description
Zinc selenide (ZnSe) is an II-VI compound semiconductor material with a face-centred cubic crystal structure and excellent physicochemical properties, which has a wide range of transmittance, from 0.5 microns to 19 microns. Zinc selenide is a very good infrared material, mainly used in infrared optics and optoelectronics, and can be used to manufacture window materials, focusing lenses, beam splitters, prisms, and CO2 lasers. Zinc selenide is also commonly used as lenses and windows for CO2 lasers due to its good imaging and thermal shock properties.
Chemical properties
Zinc selenide(ZnSe) is a yellow shiny fine powder that rarely occurs in nature. It is a chemically inert, non-hygroscopic and highly pure material largely found in the mineral stilleite. The transmissivity of zinc selenide is relatively constant over the wavelength range between 0.7 × 10 -6 to 16 × 10 -6 m, making it an excellent choice for flare radiation measurement. ZnSe is highly transparent to the radiation emitted from a flare flame. It is effective in many optical applications owing to its high resistance to thermal shock, stability in virtually all environments and extremely low bulk losses.
The Uses of Zinc selenide
Zinc selenide (ZnSe) is used as an infrared optical material with wide transmission wavelength range. It is also used as an entrance optic in the new range of "in-ear" clinical thermometers. ZnSe is used as a semiconductor material for thin film devices, and n-type windows layer for thin film heterojunction solar cells. ZnSe activated with tellurium is a scintillator suitable for matching with photodiodes. It is used in x-ray and gamma ray detectors. Zinc selenide films are useful in photovoltaic cells and solar conversion cells. The material can be doped with n-type and p-type doping. It is used to form light-emitting diodes and diode lasers. ZnSe doped with chromium finds use as an IR laser gain medium.
Air & Water Reactions
ZnSe is insoluble in water, but reacts with acids to form toxic hydrogen selenide gas. It can be deposited as a thin film by chemical vapour deposition techniques including MOVPE and vacuum evaporation.
Hazard
Fire risk in contact with water or acids.
Synthesis
Zinc Selenide is produced by synthesis from Zinc vapour and H2Se gas, forming as sheets on Graphite susceptors. Another method of producing is a growth from melt under excessive pressure of inert gas (Ar usually).
References
[1] HYUN SEON HONG. Facile synthesis and characterization of zinc selenide nanoparticles in aqueous solution at room temperature[J]. Journal of Crystal Growth, 2020, 535: Article 125523. DOI:10.1016/j.jcrysgro.2020.125523.
[2] HILE D D, SWART H C, MOTLOUNG S V, et al. Zinc selenide semiconductor: synthesis, properties and applications[J]. Nanoscale Compound Semiconductors and their Optoelectronics Applications, 2022. DOI:10.1016/b978-0-12-824062-5.00001-4.
[3] https://www.crystran.co.uk/optical-materials/zinc-selenide-znse
Properties of Zinc selenide
| Melting point: | 1100 °C |
| Density | 5.42 g/mL at 25 °C(lit.) |
| solubility | insoluble in H2O; soluble in dilute acid solutions |
| form | random pieces |
| color | Yellow |
| Specific Gravity | 5.42 |
| Water Solubility | Insoluble in water. Decomposes in dilute nitric acid. |
| Sensitive | Air Sensitive |
| Merck | 14,10156 |
| Solubility Product Constant (Ksp) | pKsp: 25.44 |
| Exposure limits | ACGIH: TWA 0.2 mg/m3 NIOSH: IDLH 1 mg/m3; TWA 0.2 mg/m3 |
| CAS DataBase Reference | 1315-09-9(CAS DataBase Reference) |
| EPA Substance Registry System | Zinc selenide (ZnSe) (1315-09-9) |
Safety information for Zinc selenide
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for Zinc selenide
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Zinc selenide CAS 1315-09-9View Details
Zinc selenide CAS 1315-09-9View Details
1315-09-9 -
 Zinc selenide CAS 1315-09-9View Details
Zinc selenide CAS 1315-09-9View Details
1315-09-9 -
 Zinc selenide CAS 1315-09-9View Details
Zinc selenide CAS 1315-09-9View Details
1315-09-9 -
 Zinc selenide CAS 1315-09-9View Details
Zinc selenide CAS 1315-09-9View Details
1315-09-9 -
 Zinc selenide CAS 1315-09-9View Details
Zinc selenide CAS 1315-09-9View Details
1315-09-9 -
 Zinc selenide CASView Details
Zinc selenide CASView Details -
 Zinc selenide CASView Details
Zinc selenide CASView Details -
 Zinc selenide CASView Details
Zinc selenide CASView Details