Chromium(III) oxide
Synonym(s):Chromia
- CAS NO.:1308-38-9
- Empirical Formula: Cr2O3
- Molecular Weight: 151.99
- MDL number: MFCD00010949
- EINECS: 215-160-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:20
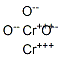
What is Chromium(III) oxide?
Description
Chromium(III) oxide is among the ten most abundant compounds in the Earth's crust. It is one of four oxides of chromium, chemical formula Cr2O3. It is commonly called "chrome green" when used as a pigment; however it was referred to as “viridian” when it was first discovered.

Chromium(III) oxide is a very refractory ceramic colorant (even a 50% mix with a high borax frit will not even begin to melt it in a crucible). Chrome oxide is the only stable oxide of the metal chromium. It is a bright to dark green crystalline powder insoluble in alkalis and acids. It is manufactured from the mineral Chromite mined in southern Africa, Asia, Turkey and Cuba. As with other powerful coloring agents, chrome must be milled fine enough to eliminate specking in glass or glaze.
Chromium is a "fast" colorant, meaning can produce strong green colors under all furnace conditions, slow or fast, reducing or oxidizing. It is also a flat colorant (due to its refractory nature), it usually produces an army helmet opaque green. It is powerful, typically only 2% will produce a dark color. It cannot be used to make a metallic glaze.
Chrome oxide is usually employed in raw glazes whereas potassium dichromate is used in fritted glazes.
Chemical properties
Chromium oxide is a bright green, odorless powder. Chromium(III) oxide pigments are thermally stable and insoluble in water.
Chromium oxide pigments, also called chromium oxide green pigments, consist of chromium(III) oxide [1308-38-9], Cr2O3,Mr 151.99. Chromium oxide green is one of the few single-component pigments with green coloration. Chrome green is a blend of chrome yellow and iron blue pigments; phthalochrome green is a blend of chrome yellow and blue phthalocyanine pigments.
Alkali dichromates are used as starting materials for the production of chromium(III) oxide pigments. They are not classified as hazardous materials and are not subject to international transport regulations. As long as they are kept dry their utility as a pigment is practically unlimited.
Physical properties
Green hexagonal crystal system; corundum type structure; density 5.22 g/cm3; melts at 2,330°C; vaporizes above 3,000°C; insoluble in water and alcohol.
The Uses of Chromium(III) oxide
Chromium(III) oxide is used as pigment for coloring green on glass and fabrics. Other important applications are in metallurgy; as a component of refractory bricks, abrasives and ceramics; and as a catalyst in hydrogenation, hydrogenolysis and many other organic conversion reactions. It also is used to prepare other chromium salts.
The Uses of Chromium(III) oxide
In abrasives, refractory materials, electric semiconductors; as pigment, particularly in coloring glass; in alloys; printing fabrics and banknotes; as catalyst for organic and inorganic reactions.
The Uses of Chromium(III) oxide
Chromium oxide (Cr2O3) is a dull green synthetic inorganic pigment, which can be used in all types of paint systems where high chemical resistance and outstanding light-fastness are required.
What are the applications of Application
Chromium(III) oxide is Suitable for vacuum deposition
Preparation
Chromium(III) oxide can be obtained by thermal decomposition of ammonium dichromate. Above ca. 200 °C, a highly voluminous product is formed with elimination of nitrogen. The pigment is obtained after addition of alkali salts (e.g., sodium sulfate) and subsequent calcination.
In the industrial process, a mixture of ammonium sulfate or chloride and sodium dichromate is calcined:
Na2Cr2O7.2 H2O + (NH4)2SO4 →Cr2O3 + Na2SO4 + 6 H2O + N2
Definition
chromium sesquioxide: A green crystallinewater-insoluble salt, Cr2O3; r.d. 5.21;m.p. 2435°C; b.p. 4000°C. It is obtainedby heating chromium in astream of oxygen or by heating ammoniumdichromate. The industrialpreparation is by reduction ofsodium dichromate with carbon.Chromium(III) oxide is amphoteric,dissolving in acids to give chromium(III) ions and in concentratedsolutions of alkalis to give chromites.It is used as a green pigment in glass,porcelain, and oil paint.
Definition
Chromium oxide (Cr2O3) also called green rouge is a dull yellowish-green pigment that may be prepared by blending an alkali dichromate with sulfur or with a carbonaceous material. Reduction to chrome (III) oxide is achieved in a kiln at 1000°C. And it is used chiefly for platinum and stainless steels.
Reactions
Chromium(III) oxide is amphoteric. Although insoluble in water, it dissolves in acid to produce hydrated chromium ion, [Cr(H2O)6]3+. It dissolves in concentrated alkali to yield chromite ion. When heated with finely divided aluminum or carbon it is reduced to chromium metal:
Cr2O3 + 3Al2Cr + Al2O3
Heating with chlorine and carbon yields chromium(III) chloride:
Cr2O3 + 3Cl2 + 3C2CrCl3 + 3CO
If chromium(III) oxide (also known as chrome green) is heated with potassium carbonate and potassium nitrate, the mixture slowly turns yellow. This colour change stems from the formation of potassium chromate, K2CrO4, in which chromium is found in oxidation state vi.
General Description
Chromium (III) oxide is a chromium complex in which the chromium ion is in +3 oxidation state. The synthesis of its sub-micron powder has been reported.? The IR and Raman spectra of chromium (III) oxide have been studied.? The impact of adding lysozyme on the stability of chromium (III) oxide (Cr2O3) suspension has been evaluated.
Hazard
Toxic by ingestion and inhalation.
Flammability and Explosibility
Not classified
Safety Profile
Confirmed carcinogen with experimental tumorigenic data. Mutation data reported. Probably a severe
Toxicology
Chromium(III) oxide, which forms the basis of chromium oxide pigments, crystallizes in a corundum lattice. Chromium oxide green pigments (Cr2O3, C.I. Pigment Green 17) contain only trivalent chrome. Acute toxicity: rat, oral, LD50>10 000 mg/kg.
Potential Exposure
Chromium(III) oxide is used as a paint pigment, a fixative for certain textile dyes; in the manufacture of chromium; and a catalyst.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately.If this chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPRif heart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, getmedical attention. Give large quantities of water andinduce vomiting. Do not make an unconscious personvomit.
Storage
(1) Color Code—Blue: Health Hazard/Poison:Store in a secure poison location. (2) Color Code—Yellow:Reactive Hazard; Store in a location separate from othermaterials, especially flammables and combustibles.Chromium(III) oxide must be stored to avoid contact withstrong oxidizers (such as chlorine, bromine, and fluorine),glycerol, and oxygen difluoride, since violent reactionsoccur. A regulated, marked area should be establishedwhere chromium(III) oxide is handled, used, or stored.Store in tightly closed containers in a cool, well-ventilatedarea.
Shipping
UN3086 Toxic solids, oxidizing, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, 5.1-Oxidizer. Technical Name Required. Spill Handling: Evacuate persons not wearing protective equipment from the danger area of spill or leak until cleanup is complete. Remove all ignition sources. Collect powdered material in the most convenient and safe manner and deposit in sealed containers. Ventilate area after clean-up is complete. It may be necessary to contain and dispose of this chemical as a hazardous waste. If material or contaminated runoff enters waterways, notify downstream users of potentially contaminated waters. Contact your local or federal environmental protection agency for specific recommendations. If employees are required to clean-up spills, they must be properly trained and equipped. OSHA 1910.120(q) may be applicable.
Properties and Applications
|
TEST ITEMS |
SPECIFICATION |
|
APPEARANCE |
GREEN POWDER |
|
CONTENT OF Cr 2 O 3 |
85% min |
|
SHADE |
YELLOWISH |
|
HYDROTROPE |
0.5% max |
|
OIL ABSORPTION |
25% max |
|
RESIDUE ON 45 MESH |
0.1% max |
|
WATER SOLUBLE |
0.3% max |
|
VOLATITE 105 °C |
0.05% max |
|
TINTING STRENGTH |
95-100 % |
|
pH VALUE OF AQUEOUS SUSPENSION |
5-8 |
Structure and conformation
Chromium oxide is a dense, crystalline material, density 5.2 g/cm3, of the corundum type. It is quite hard-abut 9 on the Moh scale-which makes it a good grinding material, but too abrasive for many pigment applications. The particle size depends on the manufacturing process, but it is distributed around mean values in the range 0.5-0.6 μm. The refractive index of chromium oxide is quite high, 2.5, which is almost as high as that of rutile TiO2, 2.7. Chromium oxide pigments are green with an olive green tint. Small particles are lighter green with a yellowish hue, whereas larger particles are darker, with a bluish tint. Chromium oxide pigments are very inert materials with outstanding lightfastness and excellent resistance to acids, alkalis, and high temperatures.
Incompatibilities
A strong oxidizer. Contact with reducing agents; organics, and combustibles may be violent
Properties of Chromium(III) oxide
| Melting point: | 2435 °C |
| Boiling point: | 4000 °C |
| Density | 5.21 |
| refractive index | 2.551 |
| Flash point: | 3000°C |
| storage temp. | Room Temperature |
| solubility | Insoluble in all solvents |
| form | powder |
| color | Pale to dark green |
| Specific Gravity | 5.22 |
| Odor | at 100.00?%. odorless |
| Water Solubility | Insoluble |
| Crystal Structure | Trigonal |
| Merck | 14,2234 |
| Exposure limits | NIOSH: IDLH 25 mg/m3; TWA 0.5 mg/m3 |
| Stability: | Stable. |
| CAS DataBase Reference | 1308-38-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Chromium(iii) oxide(1308-38-9) |
| EPA Substance Registry System | Chromium(III) oxide (1308-38-9) |
Safety information for Chromium(III) oxide
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P321:Specific treatment (see … on this label). P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for Chromium(III) oxide
Chromium(III) oxide manufacturer
JSK Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 CHROMIUM OXIDE GREEN 99%View Details
CHROMIUM OXIDE GREEN 99%View Details -
 CHROMIUM TRIOXIDE GREEN 99%View Details
CHROMIUM TRIOXIDE GREEN 99%View Details -
 Chromium oxide CAS 1308-38-9View Details
Chromium oxide CAS 1308-38-9View Details
1308-38-9 -
 Chromium oxide CAS 1308-38-9View Details
Chromium oxide CAS 1308-38-9View Details
1308-38-9 -
 Chromium(III) oxide CAS 1308-38-9View Details
Chromium(III) oxide CAS 1308-38-9View Details
1308-38-9 -
 Chromium(III) oxide CAS 1308-38-9View Details
Chromium(III) oxide CAS 1308-38-9View Details
1308-38-9 -
 Chromium(III) oxide CAS 1308-38-9View Details
Chromium(III) oxide CAS 1308-38-9View Details
1308-38-9 -
 Chromium(III) oxide CAS 1308-38-9View Details
Chromium(III) oxide CAS 1308-38-9View Details
1308-38-9
