COBALT(II) BROMIDE
- CAS NO.:7789-43-7
- Empirical Formula: Br2Co
- Molecular Weight: 218.74
- MDL number: MFCD00016017
- EINECS: 232-166-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-29 14:21:09
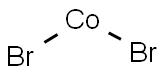
What is COBALT(II) BROMIDE?
Chemical properties
Reddish violet crystalline powder
Chemical properties
The green anhydrous salt is prepared by dehydration of the red hexahydrate or by the action of bromine on heated cobalt. It is very soluble in water and soluble in many polar organic solvents; it deliquesces in moist air to a red solution. The hexahydrate crystallizes from aqueous solution at room temperature; it melts at 100°, evolving water and forming the purple dihydrate.
The Uses of COBALT(II) BROMIDE
Cobalt(II) bromide is used as a catalyst in organic synthesis. It is used as a precursor in the production of ethylsulfanyl)porphyrazinato)cobalt(II), which gives the possibility of intermolecular ferromagnetic interactions.
The Uses of COBALT(II) BROMIDE
Used in the preparation of a new complex, Co(OESPz), which offers the possibility of intermolecular ferromagnetic interactions.1
Preparation
Cobalt(II) bromide (CoBr2) is on its anhydrous form a green solid that is soluble in water, used primarily as a catalyst in some processes. Cobalt(II) bromide can be formed as a hydrate by the reaction of cobalt hydroxide with hydrobromic acid.
Co (OH) 2 (s)+2HBr (aq)→CoBr2.6H2O(aq)
General Description
COBALT(II) BROMIDE is a red violet crystalline solid. COBALT(II) BROMIDE is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. COBALT(II) BROMIDE is used as a catalyst in the production of other chemicals.
Air & Water Reactions
Water soluble.
Reactivity Profile
Acidic salts, such as COBALT(II) BROMIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. When heated to decomposition can give off highly toxic fumes of Br [USCG, 1999].
Health Hazard
SOLID: Irritating to skin and eyes. Harmful if swallowed.
Fire Hazard
Not flammable. POISONOUS FUMES ARE PRODUCED WHEN HEATED TO DECOMPOSITION. When heated to decomposition can give off highly toxic fumes of Br.
Purification Methods
Crystallise it from water (1mL/g) by partial evaporation in a desiccator. The anhydrous salt is soluble in EtOH, Me2CO, MeOAc to form blue-coloured solutions. [Glemser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1517 1965.]
Properties of COBALT(II) BROMIDE
| Melting point: | 678°C |
| Density | 4.909 g/mL at 25 °C(lit.) |
| solubility | acetone: soluble(lit.) |
| form | beads |
| Specific Gravity | 4.909 |
| color | Green |
| Water Solubility | Soluble in water. Soluble in methyl acetate, ether, alcohol, acetone. |
| Sensitive | Hygroscopic |
| Merck | 14,2435 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3 |
| CAS DataBase Reference | 7789-43-7(CAS DataBase Reference) |
| EPA Substance Registry System | Cobalt bromide (7789-43-7) |
Safety information for COBALT(II) BROMIDE
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H334:Sensitisation, respiratory H341:Germ cell mutagenicity H350:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for COBALT(II) BROMIDE
Abamectin manufacturer
Axiom Chemicals Pvt Ltd (Axiom Corporation)
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
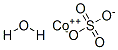
You may like
-
 7789-43-7 98%View Details
7789-43-7 98%View Details
7789-43-7 -
 Cobalt(II) bromide, Anhydrous CAS 7789-43-7View Details
Cobalt(II) bromide, Anhydrous CAS 7789-43-7View Details
7789-43-7 -
 Cobalt(II) bromide, Anhydrous CAS 7789-43-7View Details
Cobalt(II) bromide, Anhydrous CAS 7789-43-7View Details
7789-43-7 -
 Cobalt(II) bromide CAS 7789-43-7View Details
Cobalt(II) bromide CAS 7789-43-7View Details
7789-43-7 -
 Cobalt(II) bromide CAS 7789-43-7View Details
Cobalt(II) bromide CAS 7789-43-7View Details
7789-43-7 -
 Cobalt(II) bromide 99% CAS 7789-43-7View Details
Cobalt(II) bromide 99% CAS 7789-43-7View Details
7789-43-7 -
 Cobalt(II) bromide CAS 7789-43-7View Details
Cobalt(II) bromide CAS 7789-43-7View Details
7789-43-7 -
 Cobalt(II) bromide CAS 7789-43-7View Details
Cobalt(II) bromide CAS 7789-43-7View Details
7789-43-7