Cobaltous nitrate hexahydrate
Synonym(s):Cobalt(II) nitrate hexahydrate;Cobaltous nitrate;Cobaltous nitrate hexahydrate;Nitric acid, cobalt(II) salt
- CAS NO.:10026-22-9
- Empirical Formula: CoH12N2O12
- Molecular Weight: 291.03
- MDL number: MFCD00149647
- EINECS: 600-049-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
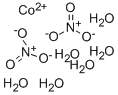
What is Cobaltous nitrate hexahydrate?
Chemical properties
Red crystal, deliquescent in most air
The Uses of Cobaltous nitrate hexahydrate
Preparation of cobalt standard solutions.
The Uses of Cobaltous nitrate hexahydrate
manufacture of cobalt pigments and invisible inks; decorating stoneware and porcelain; preparation of catalysts; production of vitamin B12 supplements.
The Uses of Cobaltous nitrate hexahydrate
Cobaltous nitrate [Co(NO3)2·6H2O], also known as cobalt nitrate, is a red crystal that absorbs moisture. It is used in inks, pigments, animal feed, soil enhancers, and hair dyes.
What are the applications of Application
Cobalt(II) nitrate hexahydrate is a salt of Co(II) that acts as a source of Cobalt
Definition
ChEBI: A hydrate that is the hexahydrate form of cobalt dinitrate.
Preparation
The red hygroscopic hexahydrate Cu(NO3)2.6H2O crystallizes from aqueous solutions of cobalt bases in dilute nitric acid at room temperature. It becomes the trihydrate when heated at 56° ; at higher temperatures decomposition occurs.
General Description
Cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O) is the hydrated cobalt salt. Its step-wise thermal degradation has been investigated. It affords anhydrous cobalt(II)nitrate as decomposition product.
Purification Methods
Crystallise the nitrate from water (1mL/g), or ethanol (1mL/g), by partial evaporation. After 3 crystallisations from H2O it contains: metal (ppm) As (8), Fe (1.2), K (1), Mg (4), Mn (4), Mo (4), Na (0.6), Ni (18), Zn (1.6). The hexahydrate gives the pink anhydrous salt by the action of HNO3 and N2O5. The hexahydrate melts at ~55o to give a red liquid which decomposes on further heating at 100-105o to form Co3O4.
Properties of Cobaltous nitrate hexahydrate
| Melting point: | 55 °C(lit.) |
| Density | 1.88 |
| refractive index | 1.52 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 2170g/l |
| form | Wet Crystalline Aggregates |
| color | Red to pinkish-brown |
| Specific Gravity | 1.87 |
| PH | 4.0 (100g/l, H2O, 20℃) |
| Water Solubility | 134 g/100 mL |
| Merck | 14,2444 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3 |
| Stability: | Stable. Strong oxidizer - incompatible with reducing agents. |
| CAS DataBase Reference | 10026-22-9(CAS DataBase Reference) |
| EPA Substance Registry System | Cobalt nitrate hexahydrate (10026-22-9) |
Safety information for Cobaltous nitrate hexahydrate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H302:Acute toxicity,oral H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H334:Sensitisation, respiratory H341:Germ cell mutagenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Cobaltous nitrate hexahydrate
| InChIKey | QGUAJWGNOXCYJF-UHFFFAOYSA-N |
Cobaltous nitrate hexahydrate manufacturer
Svaks Biotech India Pvt Ltd
S D Fine Chem Limited
ARRAKIS INDUSTRIES LLP
Anand Agencies
New Products
1-Amino-1-cyclohexanecarboxylic acid Cycloleucine 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole ELECTROLYTIC IRON POWDER 2-Methyl-2-phenylpropyl acetate 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate 4-Ethylbenzylamine Methyl 5-bromo-2-chloro-3-nitrobenzoate N-(5-Amino-2-methylphenyl)acetamide 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 5-Fluoro-2-Oxindole methyl L-alaninate hydrochloride diethyl L-glutamate hydrochloride Ethyl tosylcarbamateRelated products of tetrahydrofuran

You may like
-
 Cobalt(II) nitrate 98%View Details
Cobalt(II) nitrate 98%View Details -
 Cobaltous nitrate hexahydrate 98%View Details
Cobaltous nitrate hexahydrate 98%View Details -
 Cobalt(II) nitrate hexahydrate CAS 10026-22-9View Details
Cobalt(II) nitrate hexahydrate CAS 10026-22-9View Details
10026-22-9 -
 Cobalt(II) nitrate hexahydrate CAS 10026-22-9View Details
Cobalt(II) nitrate hexahydrate CAS 10026-22-9View Details
10026-22-9 -
 Cobalt(II) nitrate hexahydrate CAS 10026-22-9View Details
Cobalt(II) nitrate hexahydrate CAS 10026-22-9View Details
10026-22-9 -
 Cobaltous nitrate hexahydrate 99%View Details
Cobaltous nitrate hexahydrate 99%View Details -
 Cobalt(II) nitrate hexahydrate CAS 10026-22-9View Details
Cobalt(II) nitrate hexahydrate CAS 10026-22-9View Details
10026-22-9 -
 Cobalt(II) nitrate hexahydrate CAS 10026-22-9View Details
Cobalt(II) nitrate hexahydrate CAS 10026-22-9View Details
10026-22-9