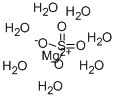
Cobalt sulfate heptahydrate
Synonym(s):Cobalt(II) sulfate heptahydrate;Cobaltous sulfate heptahydrate
- CAS NO.:10026-24-1
- Empirical Formula: CoH14O11S
- Molecular Weight: 281.1
- MDL number: MFCD00149658
- EINECS: 600-050-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
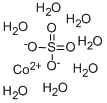
What is Cobalt sulfate heptahydrate?
Chemical properties
Cobalt sulphate appears as pink or red crystals. It is stable, non-flammable, and hygroscopic and undergoes dehydration at 41°C and 71°C.
Chemical properties
Red powder or rose pink crystalline solid. Odorless.
The Uses of Cobalt sulfate heptahydrate
Usual source of water-soluble cobalt since it is the most economical and it shows less tendency to deliquesc or dehydrate than the chloride or nitrate. Used in storage batteries; in Co-electroplating baths; as drier for lithographic inks, varnishes; in ceramics, enamels, glazes to prevent discoloring; in Co pigments for decorating porcelain.
The Uses of Cobalt sulfate heptahydrate
Cobalt(II) sulfate heptahydrate is used in the preparation of pigments and other cobalt salts. It is used in storage batteries and electroplating baths, sympathetic inks, and as an additive to soils and animal feeds.
Definition
ChEBI: A hydrate that is the heptahydrate form of cobalt(2+) sulfate.
General Description
Pink to red monoclinic prismatic crystals or red granular solid. Odorless. Becomes anhydrous at 788°F.
Air & Water Reactions
Water soluble. The resulting solution is acidic.
Reactivity Profile
Does not react strongly as either oxidizing agents or reducing agents but such behavior is not impossible. The aqueous solutions are acidic. May catalyze organic reactions.
Fire Hazard
Literature sources indicate that Cobalt sulfate heptahydrate is nonflammable.
Potential Exposure
Many be used to catalyze organic reactions.
Shipping
UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Crystallise it three times from conductivity water (1.3mL/g) between 100o and 0o depending on which hydrate is required. The heptahydrate crystallises below 44o and is efflorescent with m 97o . Between 44o and 70o the monoclinic hexahydrate CoSO4.6H2O m 41.5o is formed, and above 70o the monohydrate CoSO4.H2O m 71o is obtained. The pale reddish or lavender-coloured anhydrous salt is obtained by heating the hydrate above 250o, boiling with conc H2SO4 or heating with (NH4)2SO4).
Incompatibilities
Aqueous solution reacts with bases, generating some heat. May react as either oxidizing agents or reducing agents
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. All federal, state, and local environmental regulations must be observed.
Properties of Cobalt sulfate heptahydrate
| Melting point: | 98 °C |
| Boiling point: | 735°C |
| Density | 2.03 g/mL at 25 °C(lit.) |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: soluble |
| form | Solid |
| color | Red-brown |
| Specific Gravity | 1.948 |
| PH | 4 (100g/l, H2O, 20℃) |
| Water Solubility | 362 g/L (20 ºC) |
| Merck | 14,2448 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3 |
| Stability: | Stable. Non-flammable. Hygroscopic. Dehydrates at around 41 C and 71 C. |
| CAS DataBase Reference | 10026-24-1(CAS DataBase Reference) |
| IARC | 2B (Vol. 86) 2006 |
| EPA Substance Registry System | Cobalt(2+) sulfate heptahydrate (10026-24-1) |
Safety information for Cobalt sulfate heptahydrate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H341:Germ cell mutagenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Cobalt sulfate heptahydrate
| InChIKey | MEYVLGVRTYSQHI-UHFFFAOYSA-L |
Cobalt sulfate heptahydrate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 10026-24-1 Cobaltous sulphate heptahydrate 98%View Details
10026-24-1 Cobaltous sulphate heptahydrate 98%View Details
10026-24-1 -
 Cobalt(II) sulfate heptahydrate CAS 10026-24-1View Details
Cobalt(II) sulfate heptahydrate CAS 10026-24-1View Details
10026-24-1 -
 Cobalt(II) sulfate heptahydrate CAS 10026-24-1View Details
Cobalt(II) sulfate heptahydrate CAS 10026-24-1View Details
10026-24-1 -
 Cobalt (II) Sulphate Heptahydrate extrapure AR CAS 10026-24-1View Details
Cobalt (II) Sulphate Heptahydrate extrapure AR CAS 10026-24-1View Details
10026-24-1 -
 Cobalt sulphate CAS 10026-24-1View Details
Cobalt sulphate CAS 10026-24-1View Details
10026-24-1 -
 Cobalt sulphate, GR 99%+ CAS 10026-24-1View Details
Cobalt sulphate, GR 99%+ CAS 10026-24-1View Details
10026-24-1 -
 COBALT (II) SULPHATE HEPTAHYDRATE Extra Pure CAS 10026-24-1View Details
COBALT (II) SULPHATE HEPTAHYDRATE Extra Pure CAS 10026-24-1View Details
10026-24-1 -
 Cobaltous Sulphate Heptahydrate, 10026-24-1View Details
Cobaltous Sulphate Heptahydrate, 10026-24-1View Details
10026-24-1
