Cobalt(II) hydroxide
Synonym(s):Cobalt dihydroxide;Cobalt(2+) hydroxide;Cobaltous hydroxide
- CAS NO.:21041-93-0
- Empirical Formula: CoH2O2
- Molecular Weight: 92.95
- MDL number: MFCD00016015
- EINECS: 244-166-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
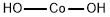
What is Cobalt(II) hydroxide?
Description
The addition of alkali metal hydroxides to solutions of cobalt(II) salts results in the precipitation of cobalt(II) hydroxide in either a blue or pink form depending upon the conditions. The pink form is the more stable of the two and is obtained when a suspension of the blue form is allowed to stand or is warmed. Cobalt(II) hydroxide is amphoteric, dissolving in alkalis to form blue solutions of the [Co(OH)4]2- ions. In the presence of alkali, suspensions of Co(OH) are oxidized by air to the brown CoO(OH), this oxidation being brought about rapidly by oxidants such as hypochlorite, bromine water or hydrogen peroxide. The pink Co(OH)2 (density 3.597) has the brucite (Mg(OH)2) structure in which the cobalt atoms are surrounded by six hydroxides. The blue form is more disordered, but its structure is not known for certain.
Chemical properties
Rose-red powder.Soluble in acids and ammonium salt solutions; insoluble in water and alkalies.
The Uses of Cobalt(II) hydroxide
Cobalt(II) hydroxide is used as a drying agent for paints and varnishes. It acts as an aid to dry the lithographic inks and catalyst in the preparation of battery electrode impregnating solutions. It is also involved in the synthesis of other cobalt compounds.
The Uses of Cobalt(II) hydroxide
Cobalt salts, paint and varnish driers, catalyst, storage-battery electrodes.
What are the applications of Application
Cobalt(II) hydroxide is a drying agent, catalyst, and precursor to other cobalt compounds
Flammability and Explosibility
Not classified
Properties of Cobalt(II) hydroxide
| Melting point: | °Cd ec.) |
| Density | 3.597 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | slightly soluble in H2O; soluble in acid solutions |
| form | Powder |
| color | Pink to purple |
| Specific Gravity | 3.597 |
| PH | 9.15(1 mM solution);9.15(10 mM solution);9.15(100 mM solution) |
| Water Solubility | Soluble in acids and ammonia. Very slightly soluble in water. Insoluble in dilute alkalis. |
| Sensitive | Air Sensitive |
| Merck | 14,2442 |
| Solubility Product Constant (Ksp) | pKsp: 14.23 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3 |
| Stability: | Stability |
| CAS DataBase Reference | 21041-93-0(CAS DataBase Reference) |
| EPA Substance Registry System | Cobalt hydroxide (Co(OH)2) (21041-93-0) |
Safety information for Cobalt(II) hydroxide
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H334:Sensitisation, respiratory H350:Carcinogenicity H360:Reproductive toxicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cobalt(II) hydroxide
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Cobalt(II) hydroxide CAS 21041-93-0View Details
Cobalt(II) hydroxide CAS 21041-93-0View Details
21041-93-0 -
 Cobalt(II) hydroxide CAS 21041-93-0View Details
Cobalt(II) hydroxide CAS 21041-93-0View Details
21041-93-0 -
 Cobalt(II) hydroxide CAS 21041-93-0View Details
Cobalt(II) hydroxide CAS 21041-93-0View Details
21041-93-0 -
 Cobalt hydroxide 95% CAS 21041-93-0View Details
Cobalt hydroxide 95% CAS 21041-93-0View Details
21041-93-0 -
 Cobalt(II) hydroxide, 95% CAS 21041-93-0View Details
Cobalt(II) hydroxide, 95% CAS 21041-93-0View Details
21041-93-0 -
 Cobalt(II) hydroxide CAS 21041-93-0View Details
Cobalt(II) hydroxide CAS 21041-93-0View Details
21041-93-0 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1