COBALT(III) FLUORIDE
Synonym(s):Cobalt trifluoride;Cobaltic fluoride
- CAS NO.:10026-18-3
- Empirical Formula: CoF3
- Molecular Weight: 115.93
- MDL number: MFCD00010942
- EINECS: 233-062-4
- Update Date: 2025-09-25 17:15:13
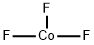
What is COBALT(III) FLUORIDE?
Chemical properties
brown powder
Chemical properties
The heat of formation is —187 kcal mole-1 and the magnetic moment is 1-60
B.M. at 90°K rising to 2.46 B.M. at 293°K; it is thus a high spin compound but not magnetically dilute.
Anhydrous cobalt(III) fluoride turns dark in the presence of traces of water and reacts violently with the liquid-evolving oxygen. When heated in an inert atmosphere above 350°, cobalt(II) fluoride is formed. It reacts vigorously with many elements, e.g. Al, P, As, S, I, being reduced to cobalt(II) fluoride. It is insoluble in alcohol, ether and benzene and is a useful fluorinating agent in organic chemistry.
Physical properties
Light brown hexagonal crystal; density 3.88 g/cm3; moisture sensitive; stable in dry air; melts at 927°C; reacts with water.
The Uses of COBALT(III) FLUORIDE
Cobalt(III) fluoride is a powerful fluorinating agent.
The Uses of COBALT(III) FLUORIDE
Important fluorinating agent, particularly for complete fluorination of hydrocarbons by the Fowler process.
What are the applications of Application
Cobalt(III) fluoride is a powerful fluorinating agent
Preparation
Cobalt(III) fluoride may be prepared by reaction of elemental fluorine with cobalt(II) fluoride, cobalt(II) chloride or cobalt(III) oxide at 300 to 400°C.
2CoF2 + F2 → 2CoF3
2CoCl2 + 3F2 → 2CoF3 + 2Cl2
It should be stored in a sealed glass ampule, free from moisture.
Electrolytic oxidation of cobalt(II) fluoride in 40% hydrofluoric acid yields hydrated cobalt(III) fluoride, CoF3·3.5H2O (3.5 is the stoichiometric amount of water per CoF3 molecule in the crystal lattice).
Hazard
Strong irritant to tissue.
Structure and conformation
It forms hexagonal crystals of density 3.89, isomorphous with iron(III) and aluminium(III) fluorides, the cobalt atoms being octahedraily surrounded by fluorine atoms.
Properties of COBALT(III) FLUORIDE
| Melting point: | 927°C |
| Density | 3.88 g/mL at 25 °C(lit.) |
| solubility | reacts with H2O; soluble in ethanol,ethyl ether, benzene |
| form | Powder |
| color | Light brown |
| Specific Gravity | 3.88 |
| Water Solubility | Insoluble in water. |
| Sensitive | Hygroscopic |
| Merck | 14,2430 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3; TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 10026-18-3(CAS DataBase Reference) |
| EPA Substance Registry System | Cobalt fluoride (CoF3) (10026-18-3) |
Safety information for COBALT(III) FLUORIDE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for COBALT(III) FLUORIDE
COBALT(III) FLUORIDE manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Cobalt(III) fluoride CAS 10026-18-3View Details
Cobalt(III) fluoride CAS 10026-18-3View Details
10026-18-3 -
 Cobalt(III) fluoride CAS 10026-18-3View Details
Cobalt(III) fluoride CAS 10026-18-3View Details
10026-18-3 -
 Cobalt(III) fluoride 99%View Details
Cobalt(III) fluoride 99%View Details
10026-18-3 -
 Cobalt(iii) fluoride 95% CAS 10026-18-3View Details
Cobalt(iii) fluoride 95% CAS 10026-18-3View Details
10026-18-3 -
 Cobalt trifluoride CAS 10026-18-3View Details
Cobalt trifluoride CAS 10026-18-3View Details
10026-18-3 -
 Cobalt(III) fluoride CAS 10026-18-3View Details
Cobalt(III) fluoride CAS 10026-18-3View Details
10026-18-3 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
