Cobalt sulfate
- CAS NO.:10124-43-3
- Empirical Formula: CoO4S
- Molecular Weight: 155
- MDL number: MFCD00010943
- EINECS: 233-334-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
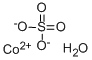
What is Cobalt sulfate ?
Description
The blue, crystalline hydrate Co2(SO4)3.18H2O is prepared by the oxidation of cobalt(II) sulphate in 8N sulphuric acid either electrolytically or chemically with ozone or fluorine. It is stable in the dry state, but is decomposed by water with evolution of oxygen; it is fairly stable in solution in dilute sulphuric acid. Cobalt(III) alums MCo(SO4)2.12H2O (M = K, Rb, Cs or NH4) can be isolated as blue crystals from the mixed cooler solutions of the two sulphates in dilute sulphuric acid. The potassium alum is diamagnetic, the rubidium salt has a magnetic moment less than 1 B.M. and the ammonium alum has a moment of 2.1 B.M. at 304°K. The hydrated sulphate also has a small positive magnetic susceptibility. The sulphate is believed like the alums to contain the [Co(H2O)6]3+ ion.
Chemical properties
Red powder or rose pink crystalline solid. Odorless.
The Uses of Cobalt sulfate
Ceramics, pigments, glazes, in plating baths for cobalt, additive to soils, catalyst, paint and ink drier, storage batteries.
The Uses of Cobalt sulfate
Cobalt(II) Sulphate is used in batteries and fuel cells that display long-term stability.
Definition
ChEBI: Cobalt(2+) sulfate is a compound of cobalt and sulfate in which the ratio of cobalt (+2 oxidation state) to sulfate is 1:1. It contains a cobalt(2+).
General Description
Odorless rose-pink solid. Sinks and mixes with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Acidic salts, such as Cobalt sulfate , are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Hazard
May not be used in food products (FDA). Possible carcinogen.
Health Hazard
Inhalation causes shortness of breath and coughing; permanent disability may occur. Ingestion causes pain and vomiting. Contact with eyes or skin causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Toxic cobalt oxide fumes may form in fire.
Flammability and Explosibility
Not classified
Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion. Questionable carcinogen. When heated to decomposition it emits toxic fumes of SOx See also COBALT COMPOUNDS.
Potential Exposure
Many be used to catalyze organic reactions.
Carcinogenicity
Cobalt sulfate is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
Shipping
UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Crystallise it three times from conductivity water (1.3mL/g) between 100o and 0o depending on which hydrate is required. The heptahydrate crystallises below 44o and is efflorescent with m 97o . Between 44o and 70o the monoclinic hexahydrate CoSO4.6H2O m 41.5o is formed, and above 70o the monohydrate CoSO4.H2O m 71o is obtained. The pale reddish or lavender-coloured anhydrous salt is obtained by heating the hydrate above 250o, boiling with conc H2SO4 or heating with (NH4)2SO4).
Incompatibilities
Aqueous solution reacts with bases, generating some heat. May react as either oxidizing agents or reducing agents
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. All federal, state, and local environmental regulations must be observed.
Properties of Cobalt sulfate
| Melting point: | decomposes at 1140℃ [JAN85] |
| Density | d425 3.71 |
| vapor pressure | 0Pa at 20℃ |
| solubility | H2O: soluble |
| form | red orthorhombic crystals |
| color | red orthorhombic crystals, crystalline |
| Water Solubility | dissolves slowly in boiling H2O [MER06]; g/100g solution H2O: 19.7 ± 0.1 (0°C), 27.2 ± 0.1 (25°C), 27.8 (100°C); solid phase, CoSO4 · 7H2O (0°C, 25°C), CoSO4 ·H2O (100°C) [KRU93] |
| Merck | 13,2473 |
| CAS DataBase Reference | 10124-43-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Cobalt sulfate(10124-43-3) |
| EPA Substance Registry System | Cobalt(II) sulfate (10124-43-3) |
Safety information for Cobalt sulfate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H290:Corrosive to Metals H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H341:Germ cell mutagenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Cobalt sulfate
Cobalt sulfate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 COBALT SULPHATE 99%View Details
COBALT SULPHATE 99%View Details -
 10124-43-3 98%View Details
10124-43-3 98%View Details
10124-43-3 -
 10124-43-3 Cobalt(II) sulfate 98%View Details
10124-43-3 Cobalt(II) sulfate 98%View Details
10124-43-3 -
 10124-43-3 98%View Details
10124-43-3 98%View Details
10124-43-3 -
 10124-43-3 Cobalt(II) sulfate 98%View Details
10124-43-3 Cobalt(II) sulfate 98%View Details
10124-43-3 -
 10124-43-3 98%View Details
10124-43-3 98%View Details
10124-43-3 -
 Powder CoH2O5S Cobalt Sulphate Pure, For LaboratoryView Details
Powder CoH2O5S Cobalt Sulphate Pure, For LaboratoryView Details
10124-43-3 -
 Cobalt Sulphate (10124-43-3 )View Details
Cobalt Sulphate (10124-43-3 )View Details
10124-43-3
