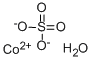CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Cobalt sulfate is an odorless rose-pink solid. Sinks and mixes with water. (USCG, 1999) |
|---|---|
| Color/Form | Red to lavender dimorphic, othorhombic crystals |
| Odor | Odorless |
| Melting Point | 735 °C |
| Solubility | Odorless; heat of solution: 23 BTU/lb= 13 cal/g= 0.54X10+5 J/kg; solubility: 35.040 lb/100 lb water at 70 °F /Cobaltous sulfate heptahydrate/ |
| Density | 1.948 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Stability/Shelf Life | Stable to 708 °C. |
| Decomposition | When heated to decomposition it emits toxic fumes of SOx |
| pH | Acidic salts, such as cobalt sulfate ... resulting aqueous solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. |
| Relative Evaporation Rate | Evaporation at 20 °C is negligible |
| Other Experimental Properties | Stable to 708 °C |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H290:Corrosive to Metals H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H341:Germ cell mutagenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 155.00 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 154.884923 g/mol |
| Monoisotopic Mass | 154.884923 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Cobalt sulfate is an odorless rose-pink solid. Sinks and mixes with water. (USCG, 1999)