Chromium(III) fluoride
Synonym(s):Chromic fluoride;Chromic trifluoride;Chromium trifluoride
- CAS NO.:7788-97-8
- Empirical Formula: CrF3
- Molecular Weight: 108.99
- MDL number: MFCD00016045
- EINECS: 232-137-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
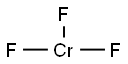
What is Chromium(III) fluoride?
Chemical properties
Green crystalline powder or crystals
Physical properties
Dark green needles (anhydrous salt) or green hexagonal crystals (trihydrate); density 3.8 g/cm3 (anhydrous fluoride), 2.2 g/cm3 (trihydrate); anhydrous salt melts at 1,100°C and sublimes above this temperature; practically insoluble in water and ethanol (anhydrous salt); trihydrate sparingly soluble in water; soluble in HCl forming a violet solution.
The Uses of Chromium(III) fluoride
Printing and dyeing woolens, moth-proofing, halogenation catalyst.
The Uses of Chromium(III) fluoride
Used in the preparation of a mixed-metal fluoride catalyst for synthesis of HFC-134a.
General Description
Chromic fluoride solution is a green crystalline solid dissolved in water. CHROMIUM(III) FLUORIDE is corrosive to metals and tissue.
Air & Water Reactions
Water soluble.
Reactivity Profile
Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas.
Hazard
Irritant to skin and eyes, especially in solution.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Not classified
Safety Profile
A poison by ingestion. A corrosive. When heated to decomposition it emits toxic vapors of Cr and F-.
Properties of Chromium(III) fluoride
| Melting point: | >1000°C |
| Density | 3.8 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 20℃ |
| solubility | insoluble in H2O, ethanol |
| form | powder |
| color | Green |
| Water Solubility | insoluble H2O; soluble HCl, violet color [MER06] |
| Sensitive | Hygroscopic |
| Merck | 14,2223 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; IDLH 25 mg/m3; TWA 0.5 mg/m3 |
| CAS DataBase Reference | 7788-97-8(CAS DataBase Reference) |
| EPA Substance Registry System | Chromium fluoride (CrF3) (7788-97-8) |
Safety information for Chromium(III) fluoride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H314:Skin corrosion/irritation H318:Serious eye damage/eye irritation H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Chromium(III) fluoride
Chromium(III) fluoride manufacturer
Jayfluoride PVT LTD
ARRAKIS INDUSTRIES LLP
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 7788-97-8 Chromium fluoride 98%View Details
7788-97-8 Chromium fluoride 98%View Details
7788-97-8 -
 7788-97-8 99%View Details
7788-97-8 99%View Details
7788-97-8 -
 Chromium(III) fluoride CAS 7788-97-8View Details
Chromium(III) fluoride CAS 7788-97-8View Details
7788-97-8 -
 Chromium(III) fluoride CAS 7788-97-8View Details
Chromium(III) fluoride CAS 7788-97-8View Details
7788-97-8 -
 7788-97-8 CHROMIUM (III) FLUORIDE 99%View Details
7788-97-8 CHROMIUM (III) FLUORIDE 99%View Details
7788-97-8 -
 Chromium fluoride CAS 7788-97-8View Details
Chromium fluoride CAS 7788-97-8View Details
7788-97-8 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1