Trichloroisocyanuric acid
Synonym(s):Trichloroisocyanuric acid;TCICA;1,3,5-Trichloro-2,4,6-triazinetrione;Trichlorocyanuric acid;1,3,5-Trichlorohexahydro-1,3,5-triazine-2,4,6-trione, 1,3,5-Trichloro-1,3,5-triazine-2,4,6-trione, TCCA, Bleaching solution
- CAS NO.:87-90-1
- Empirical Formula: C3Cl3N3O3
- Molecular Weight: 232.41
- MDL number: MFCD00006553
- EINECS: 201-782-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
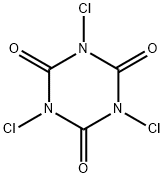
What is Trichloroisocyanuric acid?
Description
Trichloroisocyanuric acid, or more formally 1,3,5-trichloro-1,3,5-triazinane-2,4,6-trione, is a disinfectant, chlorinating agent, and industrial deodorant. It''s the active ingredient in "chlorine" tablets used to keep swimming pools safe. Its preparation was first reported by C. H. G. Hands and F. Whitt in 1948.
Chemical properties
white to light yellow crystal powde
The Uses of Trichloroisocyanuric acid
Trichloroisocyanuric Acid is a disinfectant and anti-bacterial. It can also be used in the synthesis of napthols.
The Uses of Trichloroisocyanuric acid
antiarrhythmic
The Uses of Trichloroisocyanuric acid
Chlorinating agent, disinfectant, industrial deodorant. In household cleansers. In removing oil and protein in stainless steel. In nonshrink treatment for wool. Oxidant and chlorinating reagent in organic synthesis.
What are the applications of Application
Trichloroisocyanuric acid is a useful heterocyclic building block for proteomics research
Definition
ChEBI: 1,3,5-trichloro-1,3,5-triazinane-2,4,6-trione is a member of 1,3,5-triazinanes and an organochlorine compound.
General Description
A white slightly hygroscopic crystalline powder or lump solid with a mild chlorine-like odor. Said to have 85 percent available chlorine. Decomposes at 225°C. Moderately toxic by ingestion. May irritate skin and eyes. Active ingredient in household dry bleaches. Used in swimming pools as a disinfectant .
Air & Water Reactions
Slightly hygroscopic [Hawley]. Slightly soluble in water. May react with water releasing gaseous chlorine. If mixed with a small amount of water, the concentrated solution (with pH at about 2.0) may explode due to the evolution of unstable nitrogen trichloride. (explanation in Bretherick 5th ed.)
Reactivity Profile
TRICHLORO-S-TRIAZINETRIONE, [DRY, CONTAINING > 39% AVAILABLE CHLORINE] may react vigorously with ammonium compounds or hydrated salts. Prolonged exposure to heat may result in the vigorous decomposition with the rupture of containers. Accelerates the burning of combustible materials [AAR 1991]. May reactwith alcohols to yield alkyl hypochlorites that decompose in the cold and explode on exposure to sunlight or heat. Hypochlorites of tertiary alcohols are less unstable than those of secondary or primary alcohols [NFPA 491 M. 1991].
Health Hazard
Inhalation causes sneezing and coughing. Contact with dust causes moderate irritation of eyes and itching and redness of skin. Ingestion causes burns of mouth and stomach.
Health Hazard
Moderately toxic by ingestion; causes lethargy, weakness, bleeding from stomach, andulceration; ingestion of very large quantities,probably within the range 150–100 g, maycause delayed death; as a strong oxidizer,the toxic properties of this substance showmany similarities to other powerful oxidants;injurious to liver and kidney, causing inflammation of gastrointestinal tract, liver discoloration, and kidney hyperemia as indicatefrom autopsy (Sax and Lewis 1995); the dermal toxicity is very low although an irritantto skin; irritation in rabbit’s eye form a 50mg dose in 24 hours was found to be severe.
LD50 oral (rat): 406 mg/kg.
Safety Profile
Moderately toxic to humans and experimentally by ingestion. Mildly toxic experimentally by skin contact. Human systemic effects by ingestion: ulceration or bleeding from stomach. A severe skin and eye irritant. Toxicity symptoms include emaciation, lethargy, weakness, and delayed death. Autopsy shows inflammation of gastrointestinal tract, liver discoloration, and kidney hyperemia. A powerful oxidizer. Forms an explosive product with cyanuric acid + sodmm hydroxide. Potentially violent reaction with combustible materials. When heated to decomposition it emits very toxic fumes of Cland NOx. Used to chlorinate swimming pools.
Properties of Trichloroisocyanuric acid
| Melting point: | 249-251 °C (lit.) |
| Boiling point: | 272.3±23.0 °C(Predicted) |
| Density | 2.07 |
| vapor pressure | 0.001-0.002Pa at 20-25℃ |
| Flash point: | 121°C |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Sparingly, Heated), DMSO (Sparingly) |
| form | Powder or Granules |
| pka | -4.49±0.20(Predicted) |
| color | White to almost white |
| Odor | chlorine odor |
| PH | 2.0-2.7 (10g/l, H2O, 20℃) |
| Water Solubility | 1.2 g/100mL (25 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,9641 |
| BRN | 202022 |
| Stability: | Stable. Hygroscopic, and may decompose in contact with moisture. Contact with combustible material may lead to fire. Incompatible with acids, reducing agents, water, strong oxidizing agents. |
| CAS DataBase Reference | 87-90-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Trichlorocyanuric acid(87-90-1) |
| EPA Substance Registry System | Trichloro-s-triazinetrione (87-90-1) |
Safety information for Trichloroisocyanuric acid
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Trichloroisocyanuric acid
| InChIKey | YRIZYWQGELRKNT-UHFFFAOYSA-N |
Trichloroisocyanuric acid manufacturer
JSK Chemicals
Neelam Aqua and Speciality Chem. P Ltd.
Zeel Product
ASM Organics
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran




You may like
-
 87-90-1 98%View Details
87-90-1 98%View Details
87-90-1 -
 Trichloroisocyanuric Acid CASView Details
Trichloroisocyanuric Acid CASView Details -
 Trichloroisocyanuric acid CAS 87-90-1View Details
Trichloroisocyanuric acid CAS 87-90-1View Details
87-90-1 -
 Trichloroisocyanuric acid CAS 87-90-1View Details
Trichloroisocyanuric acid CAS 87-90-1View Details
87-90-1 -
 Trichloroisocyanuric acid, 95% CAS 87-90-1View Details
Trichloroisocyanuric acid, 95% CAS 87-90-1View Details
87-90-1 -
 Trichloroisocyanuric Acid CAS 87-90-1View Details
Trichloroisocyanuric Acid CAS 87-90-1View Details
87-90-1 -
 Trichloroisocyanuric acid CAS 87-90-1View Details
Trichloroisocyanuric acid CAS 87-90-1View Details
87-90-1 -
 Trichloroisocyanuric acid CAS 87-90-1View Details
Trichloroisocyanuric acid CAS 87-90-1View Details
87-90-1
