Chloroacetaldehyde
- CAS NO.:107-20-0
- Empirical Formula: C2H3ClO
- Molecular Weight: 78.5
- MDL number: MFCD00006992
- EINECS: 203-472-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
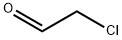
What is Chloroacetaldehyde?
Description
oroacetaldehyde is a combustible, color?less liquid with a very sharp, irritating odor. Molecularweight=78.50; Specific gravity (H2O:1)=1.19 (40%solution); Boiling point=85 100℃; Freezing/Meltingpoint=16℃(40% solution); Vapor pressure 5100 mmHg at 20℃; Flash point=87.7℃(40%626 Chloroacetaldehydesolutions). Hazard Identification (based on NFPA 704 MRating System): Health 3, Flammability 2, Reactivity 0(able to polymerize on standing). Soluble in water.
Chemical properties
Chloroacetaldehyde is a combustible, colorless liquid with a very sharp, irritating odor.
Physical properties
Clear, colorless liquid with an irritating, acrid odor
The Uses of Chloroacetaldehyde
Chloroacetaldehyde is used in the productionof 2-aminothiazole.
The Uses of Chloroacetaldehyde
In the manufacture of 2- aminothiazole; to facilitate bark removal from tree trunks; formed during the chlorination of drinking water; a metabolite of vinyl chloride
Definition
ChEBI: Chloroacetaldehyde is acetaldehyde substituted at C-2 by chlorine. It derives from an acetaldehyde.
General Description
A clear colorless liquid with a pungent odor. Flash point about 190°F. Corrosive to skin and mucous membranes. Chloroacetaldehyde is very toxic by inhalation.
Air & Water Reactions
Soluble in water. Forms an insoluble hemihydrate at greater than 50% concentration.
Reactivity Profile
Chloroacetaldehyde polymerizes on standing. At greater than 50% concentration in water, Chloroacetaldehyde forms an insoluble hemihydrate. Sensitive to heat. Reacts with oxidizing agents. Incompatible with acids and water . Burns to give poisonous and irritating gases.
Hazard
Corrosive to skin and mucous membranes. TLV: ceiling 1 ppm.
Health Hazard
Chloroacetaldehyde is a highly toxic andcorrosive compound that can injure the eyes,skin, and respiratory system. Exposure toits vapor at high concentrations can producesevere irritation and impair vision. At lowconcentrations, the vapor can cause irritationand sore eyelids. Brief contact with 40%aqueous solution can result in skin burn anddestruction of tissues. A 0.5% dilute solutioncan still be irritating on skin.
Inhalation of its vapor at the 5-ppm levelcan irritate the eyes, nose, and throat. Ingestionmay result in pulmonary edema. Swallowinga concentrated solution may be fatal.The acute toxicity data are as follows:
LD50 value, intraperitoneal (rats): 2 mg/kg
LD50 value, oral (rats): 23 mg/kg
LD50 value, skin (rabbits): 67 mg/kg
This compound is a mutagen, testing positivein the Ames test.
Fire Hazard
Combustible; flash point (closed cup) 87.8°C (190°F); flash point of 50% aqueous solution 53°C (128°F) (at this concentration it may form insoluble hemihydrate); it forms an explosive mixture with air. Reactions with strong acids and oxidizers are exothermic.
Safety Profile
Suspected carcinogen. Poison by ingestion, skin contact, and intraperitoneal routes. Mutation data reported. Combustible when exposed to heat or flame. Reacts with oxidizing materials. To fight fire, use water, foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of Cl-. See also ALDEHYDES and CHLORIDES.
Potential Exposure
Chloroacetaldehyde is used as a fungicide; as an intermediate in 2-aminothiazole manufacture; and in bark removal from tree trunks.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least30 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
Carcinogenicity
Chloroacetaldehyde has been reported to
be an inhibitor of DNA synthesis and to form
DNA adducts; it is mutagenic in Salmonella
typhimurium and in Chinese hamster cells.
Limited in vivo genotoxicity studies with
chloroacetaldehyde were negative.
Environmental Fate
Chemical/Physical. Polymerizes on standing (Windholz et al., 1983).
Storage
Polymerizable upon standing. Color Code—Blue:Health Hazard/Poison: Store in a secure poison location.Prior to working with chloroacetaldehyde you should betrained on its proper handling and storage. Store in tightlyclosed containers in a cool, dry, well-ventilated area. Metalcontainers involving the transfer of this chemical should begrounded and bonded. Where possible, automatically pumpliquid from drums or other storage containers to processcontainers. Drums must be equipped with self-closingvalves, pressure vacuum bungs, and flame arresters. Useonly nonsparking tools and equipment, especially whenopening and closing containers of this chemical. Sources ofignition, such as smoking and open flames, are prohibitedwhere this chemical is used, handled, or stored in a mannerthat could create a potential fire or explosion hazard.
Shipping
UN22322-Chloroethanal, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, Inhalation Hazard Zone B.
Incompatibilities
Heat and water sensitive; concentrations of .50% form insoluble hemihydrate material on contact with water. Reacts with oxidizers, acids. On heating,chloroacetaldehyde releases chlorine fumes. Polymerizable upon standing
Waste Disposal
Incineration, preferably after mixing with another combustible fuel; care must be exercised to assure complete combustion to prevent the formation of phosgene; an acid scrubber is necessary to remove the halo acids produced.
Properties of Chloroacetaldehyde
| Melting point: | -28--23°C |
| Boiling point: | 80-100 °C(lit.) |
| Density | 1.236 g/mL at 25 °C |
| vapor pressure | 100 at 20 °C (NIOSH, 1997) |
| refractive index | n |
| Flash point: | 128 °F |
| solubility | Soluble in ether (Weast, 1986), acetone, and methanol (Hawley, 1981) |
| form | Colorless liquid |
| Water Solubility | soluble in acetone, methanol. Fully miscible in water. |
| Sensitive | Air Sensitive |
| Merck | 2109 |
| BRN | 1071226 |
| Exposure limits | Ceiling 3 mg/m3 (1 ppm) (ACGIH); IDLH. |
| CAS DataBase Reference | 107-20-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetaldehyde, chloro-(107-20-0) |
| EPA Substance Registry System | Chloroacetaldehyde (107-20-0) |
Safety information for Chloroacetaldehyde
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Chloroacetaldehyde
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

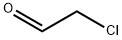


You may like
-
 Chloroacetaldehyde ~55% solution in water CAS 107-20-0View Details
Chloroacetaldehyde ~55% solution in water CAS 107-20-0View Details
107-20-0 -
 2 Chloro Acetaldehyde, 50 kg, Bio-Tech GradeView Details
2 Chloro Acetaldehyde, 50 kg, Bio-Tech GradeView Details
107-20-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
